Depression Predicts Global Functional Outcomes in Individuals at Clinical High Risk for Psychosis
Abstract
Objectives
While co‐morbid depression is associated with poor functional outcome among patients with schizophrenia, whether depression similarly predicts poorer outcomes in individuals at clinical high‐risk for psychosis (CHR‐P) is not clear. The present study aimed to examine depressive symptoms in relation to long‐term global functional outcomes in the North American Prodrome Longitudinal Study cohort (NAPLS2).
Methods
CHR individuals were evaluated clinically at baseline and at 12‐ and 24‐month follow‐ups for depressive and prodromal symptom severity as well as general functioning. Regression models were built to investigate whether baseline positive and depressive symptom scores predicted longitudinal improvement in global functioning.
Results
A total of 406 CHR individuals completed the 12‐month follow‐up assessment and 259 CHR individuals completed the 24‐month assessment. Baseline depressive symptoms in the CHR‐P population were found to predict better global functional outcomes at 2 years. Furthermore, the degree of recovery of depressive symptoms in the first year following baseline completely mediated the association between depressive symptoms at baseline and functional improvement at 2 years.
Conclusions
Presence of affective symptoms within the CHR‐P population has different implications for prognosis compared with patients with schizophrenia. The present findings support the view that among those at risk for psychosis, depressive symptoms at baseline predict a more favorable course of functional recovery, and highlight the potential importance of treating co‐occurring depressive symptoms at an early stage of psychosis risk.
Highlights
Depression among those at clinical high‐risk for psychosis is indicative of an affective subtype, that is, more likely to recover and experience better long‐term outcomes
The degree of recovery of depressive symptoms in the first year following baseline completely mediated the association between depressive symptoms at baseline and functional improvement at 2 years
This pattern highlights the potential importance of treating co‐occurring depressive symptoms at an early stage of psychosis risk
Depression is highly prevalent among those with and at risk for psychosis, with comorbidity rates ranging between 40% and 50% for individuals with a clinical high‐risk for psychosis (CHR‐P) syndrome (1, 2). Depression and psychosis also share environmental risk factors, such as childhood trauma (3), and genetic risk factors, with polygenic risk for schizophrenia also associating with affective dysregulation (4). Similarly, an elevated emotional reactivity to stress and reduced motivation or effort‐cost decision‐making (5, 6) are present in both psychosis and depression. Together, these observations suggest that depressive symptoms are highly relevant to the developmental origins of psychosis (7).
Many studies have shown a moderating effect of depression on global functional outcomes in individuals with schizophrenia (8), such that depression is indicative of worse long‐term global functional outcomes (9), including a higher risk of suicidal behavior (7) and social functioning decline (10). Depression also shares similar cognitive impairment profiles with schizophrenia (11), across domains of executive function, memory, and attention (12). In particular, there is a tendency to mislabel neutral emotion in both depression and schizophrenia, thought to be driven by the negative interpretation bias in depression (13) and impaired safety signaling in schizophrenia (14), respectively. Thus, among patients with fully psychotic forms of illness, depression appears associated with greater severity, chronicity, and functional disability. In these cases, depression may have developed as a consequence of greater psychosis disease burden. However, depression may have the opposite association in predicting global functional outcomes within the CHR‐P population. Indeed, prior research has suggested that depressive symptoms in the early stages of psychosis may be indicative of a more episodic subtype, that is, associated with better long‐term outcome (6), potentially because depressive symptoms may be responsive to treatments during this phase of illness. Theoretically, an “affective” pathway to psychosis, characterized by affective dysregulation, may be distinct from a “cognitive” pathway, which may lead to a more chronic course of illness and poorer functional outcome (15).
The present study aimed to investigate the roles of psychotic and depressive symptoms in predicting longitudinal global functional outcomes within the CHR‐P population, while accounting for the potentially confounding effects of baseline neurocognitive abilities and anxiety symptoms. Given that higher levels of attenuated positive symptoms are associated with a higher risk of conversion to psychosis, we hypothesized that higher levels of positive symptoms at baseline will predict worse longitudinal global functional outcomes. The two theoretical perspectives considered above yield opposite (mutually exclusive) predictions with regard to the association of depressive symptoms with long‐term functioning. If depressive symptoms track with greater psychosis‐related disease burden and chronicity among CHR‐P individuals as they do in patients with schizophrenia, higher depressive symptoms will predict more severe functional impairments over time (though perhaps not independently of positive symptom severity, given that this model predicts collinearity between psychotic and depressive symptoms). Alternatively, if depressive symptoms signal in line with the theorized affective pathway to psychosis among CHR‐P individuals, their presence at baseline should be predictive of better long‐term global functional outcomes, with reductions in depressive symptoms mediating improvements in functioning over time.
METHODS
Participants
The participants were recruited as a part of the second phase of North American Prodrome Longitudinal Study (NAPLS 2) (16) which was an 8‐site consortium study aiming to investigate predictors and mechanisms of conversion to psychosis. The overall sample consisted of 764 CHR participants (436 males, 328 females; ages 12–35) who met the Criteria of Psychosis‐Risk Syndromes (COPS) as determined by the Structured Interview for Psychosis‐Risk Syndromes (SIPS) (17). Exclusion criteria included any current or lifetime Axis I psychotic disorder (including affective psychoses), any clinically significant developmental or neurological disorder, and current drug or alcohol dependence.
Measures
Symptom measures
Structured Interview for prodromal Risk Syndromes (SIPS)
The SIPS (17) was used to determine whether an individual met criteria for a CHR‐P syndrome. The Scale of Psychosis‐Risk Symptoms (SOPS), used to rate the severity of symptoms, consists of 19 items in four symptom domains: positive, negative, general, and disorganized. A new subscale was made to capture symptoms reflective of depression by taking a sum of items measuring sleep disturbance, dysphoric mood, and impaired tolerance to normal stress. The items had an acceptable internal consistency (Cronbach’s alpha = 0.646).
Calgary Depression Scale for Schizophrenia (CDSS)
The CDSS (18) is a well‐established clinician‐rated measure for current depressive symptom severity over the past 2 weeks and has been validated in the CHR‐P population (19). There are nine items in the scale, each graded on a 4‐point Likert scale (0 being absent and 3 being severe). There are two sub‐scores representing general depression (e.g., guilt and hopelessness) and melancholia (e.g., early awakening). The CDSS total score is highly correlated with both the dysphoric mood item of the SOPS and the presence of a DSM‐V major depressive disorder.
Social Interaction Anxiety Questionnaire (SIAS)
The SIAS is a reliable self‐report questionnaire widely used to measure fears of general social interaction. The SIAS consists of 20 items rated on a 5‐point Likert scale (0–4), with total scores ranging from 0 (least anxiety) to 76 (most anxiety) (20).
Primary outcome measure
Global assessment of functioning (GAF)
As an overall measure of an individual’s state of wellbeing, the GAF captures the psychological, social, and occupational aspects of functioning. The GAF score ranges from 100, positive mental health to 0, severe psychopathology and functional disability. The GAF is widely used as a transdiagnostic and multidimensional measure for global functional outcomes (21). Our use of the GAF is consistent with its original purpose as a general measure of functioning encompassing both symptom‐related distress and social/role functioning dimensions (22). The GAF has previously been shown to be reliable and valid in studies of patients with psychotic disorders, with interrater reliability (intraclass correlations) ranging from 0.89 to 0.95 (21) and with GAF ratings correlating highly with external measures of work and school‐related problems and symptom‐related distress (21, 23). The GAF provides a reliable and valid global outcome metric, that is, quantitative rather than qualitative (diagnostic) in nature, encompassing a full range of outcomes related to psychiatric illnesses.
Neurocognitive ability measures.
Penn emotion recognition and differentiation task
The Penn Emotion Recognition task (ER40) and Emotion Differentiation task (EDF40) were used to assess facial affect perception and differentiation (24, 25). In these tasks, pictures displaying faces expressing different affective states are shown in color. In the ER40, participants are asked to select one emotion that best describes the face shown from five options (anger, fear, neutral, happy, and sad). In the EDF40, two faces are displayed side by side, and participants are asked to indicate which one shows an emotion (either happy or sad) more intensely. There are four faces of each gender for each emotion, and four racial/ethnic groups are represented (White, African American, Asian, and Hispanic). Both tasks have a total score ranging from 0 to 40, with sub‐scores indicating emotion recognition ability for individual emotions. Both tasks are widely used for CHR‐P individuals (26).
Measurement and treatment research to improve cognition in schizophrenia (MATRICS)
As a well‐established measure to assess neurocognitive functioning, the MATRICS cognitive battery (27) includes 10 standardized measures that evaluate 7 domains of neurocognitive ability: working memory, social cognition, verbal and visual learning, speed of processing, reasoning/problem solving, and attention. The MATRICS battery has demonstrated good internal consistency and reliability (28).
Procedures
The NAPLS‐2 study was approved by the Institutional Review Boards of all eight participating sites. Informed consent was obtained from those who met criteria and voluntarily enrolled. Parental consent was obtained from parents/guardians of minors. Participants were assigned to an experienced research clinician as the rater for the SIPS. All raters demonstrated competency on the gold standard post‐training agreement that determines a psychosis‐risk diagnosis (kappa ≥0.90) (29). Tasks measuring neurocognitive and emotional abilities were conducted at all sites by trained research assistants and post‐doctoral fellows. Following the initial assessment phase, follow‐up assessments were done every 6 months for clinical interviews and symptom measures and every 12 months for behavioral tasks (the MATRICS, ER40, and EDF40), for up to 2 years.
Statistical Analysis
Statistical analyses were performed using SPSS software version 26.0. Regression analysis was used to test whether the four symptom categories at baseline—positive and negative symptoms of psychosis, depressive symptoms, and anxiety—predicted global functioning improvement at 1 year and at 2 years. In these models, GAF improvement was expressed as the difference between the GAF score at the 1‐ or 2‐year follow‐up and the GAF score at baseline. Given the association between depression and cognitive impairments in schizophrenia, we also included two measures of cognitive functioning, baseline neutral emotion perception scores and MATRICS scores, in the regression models to account for their possible effects on longitudinal global functional outcomes. All analyses were repeated with age and sex added to the models as covariates, respectively.
To better explain the relationship between baseline depression scores and global functioning improvement, subsequent analyses examined the amount of recovery in depressive symptoms as a possible mediator using the PROCESS macro v3.3 (30). To account for the temporal sequence, the amount of recovery in depressive symptoms at 1 year was used as a mediator in examining the relationship between baseline symptom scores and global functioning improvement at 2 years. A bias‐corrected 95% bootstrap confidence interval using 5,000 bootstrap samples was used to assess for the statistical significance of the indirect effect.
A second analysis was done in parallel to affirm the robustness of the above models, using the depression sub‐score of the SOPS in place of the CDSS score. Both the linear regression and mediation analyses were repeated with all other predictors unchanged.
Lastly, to control for the possibility of a regression to the mean artifact in the primary linear regression analysis of change in GAF (given that those with lower baseline GAFs had greater room for improvement), logistic regression was performed to further investigate the baseline predictors of global functioning improvement by binarizing the outcome variables. The same predictors from the linear regression models were used to predict global functioning improvement, now as defined by an increase (i.e., by any amount) versus stability or worsening (i.e., by any amount) in the GAF score from baseline to 1 or 2 years.
RESULTS
A total of 406 CHR individuals completed the 12‐month follow‐up assessment and 259 CHR individuals completed the 24‐month assessment. The sample of participants that completed the 24‐month follow‐up did not differ from those lost to follow‐up on any demographic variable or baseline symptom score. Those who completed the 12‐month assessment had a significantly higher global functioning score (t = 2.78, p = 0.005) and lower positive symptom score (t = −2.26, p = 0.024) at baseline than those who were lost to follow‐up. The characteristics of the sample are summarized in Table 1. Cross‐sectional correlations between baseline predictors are summarized in Supplementary Figures 1–5.
| 12 months | 24 months | |||||
|---|---|---|---|---|---|---|
| Respondents | Lost to follow‐up | Test statistic | Respondents | Lost to follow‐up | Test statistic | |
| n = 406 | n = 358 | n = 259 | n = 505 | |||
| Frequency (%) | χ2 | Frequency (%) | χ2 | |||
| Sex (female) | 168 (41.38) | 160 (44.69) | 0.85 | 117 (45.17) | 211 (41.78) | 0.80 |
| Mean (SD) | t | Mean (SD) | t | |||
| Age (years) | 18.65 (4.36) | 18.33 (4.08) | 1.01 | 18.68 (4.23) | 18.41 (4.24) | 0.85 |
| Baseline positive symptoms | 11.62 (3.91) | 12.24 (3.68) | −2.26* | 11.81 (4.05) | 11.96 (3.69) | −0.50 |
| Baseline negative symptoms | 11.86 (5.94) | 11.93 (6.23) | −0.14 | 12.07 (5.95) | 11.79 (6.13) | 0.59 |
| Baseline depressive symptoms | 5.84 (4.79) | 5.80 (4.72) | 0.11 | 6.08 (4.94) | 5.68 (4.65) | 1.08 |
| Baseline anxiety symptoms | 31.38 (17.44) | 30.05 (17.36) | 1.00 | 32.03 (17.39) | 30.11 (17.40) | 1.39 |
| Baseline global functioning | 49.39 (11.36) | 47.23 (9.90) | 2.78** | 48.88 (10.67) | 48.14 (10.79) | 0.90 |
Predicting the Scale of Global Functioning Change
Assumptions of homoscedasticity and normality of residuals were met for all analyses. Collinearity statistics were within acceptable limits (VIF’s < 1.21, Tolerance’s > 0.83). In the linear regression model that included baseline affective and psychosis‐risk positive symptoms as possible predictors for global functioning improvement (Table 2), higher levels of depressive symptoms (t = 2.06, p = 0.040) and lower levels of positive symptoms (t = −2.13, p = 0.034) significantly predicted greater global functioning improvement at 1 year (R2 = 0.03). Similarly, less severe positive symptoms (t = −2.25, p = 0.005) and more depressive symptoms at baseline (t = 1.73, p = 0.024) predicted greater global functioning improvement at 2 years (R2 = 0.07). Analyses were repeated with the two sub‐scores of the CDSS—general depression and melancholia. The relation between higher depression severity and greater global functioning improvement was only present in the general depression subscale (Supplementary Table 1). To control for the baseline variations in global functioning ability, regression models were also repeated substituting percentage change from baseline for simple change scores. Significant associations between depression severity and global functioning improvements remained with percentage change scores as the outcome variable (Supplementary Table 2).
| Predictors | Dependent variable: global functioning improvement in 1 year | Dependent variable: global functioning Improvement in 2 years | ||||||
|---|---|---|---|---|---|---|---|---|
| SE | β | t | 95% CI | SE | β | t | 95% CI | |
| Positive symptoms | 0.19 | −0.11 | −2.13* | [−0.76, −0.03] | 0.21 | −0.15 | −2.25* | [−0.89, −0.06] |
| Negative symptoms | 0.13 | 0.06 | 0.98 | [−0.13, 0.39] | 0.17 | 0.08 | 1.08 | [−0.15, 0.50] |
| Anxiety | 0.05 | −0.08 | −1.26 | [−0.15, 0.03] | 0.06 | −0.11 | −1.55 | [−0.20, 0.02] |
| Depression | 0.18 | 0.12 | 2.06* | [0.02, 0.70] | 0.20 | 0.13 | 1.73† | [−0.05, 0.75] |
| Neurocognitive ability | 0.10 | 0.06 | 1.14 | [−0.08, 0.30] | 0.11 | 0.10 | 1.60 | [−0.04, 0.39] |
| Neutral emotion recognition | 0.68 | <0.01 | 0.02 | [−1.33, 1.36] | 0.86 | 0.10 | 1.65 | [−0.28, 3.12] |
| Positive symptoms | 0.18 | −0.12 | −2.26* | [−0.78, −0.05] | 0.21 | −0.15 | −2.36* | [−0.91, −0.08] |
| Negative symptoms | 0.14 | 0.02 | 0.38 | [−0.22, 0.33] | 0.17 | 0.05 | 0.61 | [−0.23, 0.44] |
| Anxiety | 0.05 | −0.07 | −1.20 | [−0.15, 0.04] | 0.06 | −0.11 | −1.60 | [−0.20, 0.02] |
| Depression (as measured in SOPS) | 0.21 | 0.17 | 2.74** | [0.16, 0.99] | 0.25 | 0.17 | 2.24* | [0.07, 1.06] |
| Neurocognitive ability | 0.10 | 0.06 | 1.20 | [−0.07, 0.31] | 0.11 | 0.11 | 1.78† | [−0.02, 0.40] |
| Neutral emotion recognition | 0.68 | 0.01 | 0.17 | [−1.23, 1.46] | 0.86 | 0.12 | 1.83† | [−0.12, 3.29] |
To better understand the relationship between higher baseline depression and greater global functioning improvement, subsequent analyses examined the amount of recovery in depressive symptoms at 12‐month as a possible mediator of functional improvement at 24‐month. The mediation model showed that the amount of recovery in depressive symptoms over the first year significantly mediated the relationship between baseline depression and global functioning improvement at 2 years (Figure 1A; 95% CI [0.29, 0.91]). Depression, however, did not mediate the inverse relationship between baseline positive symptoms and functional outcome at 2 years (95% CI [−0.07, 0.14]).
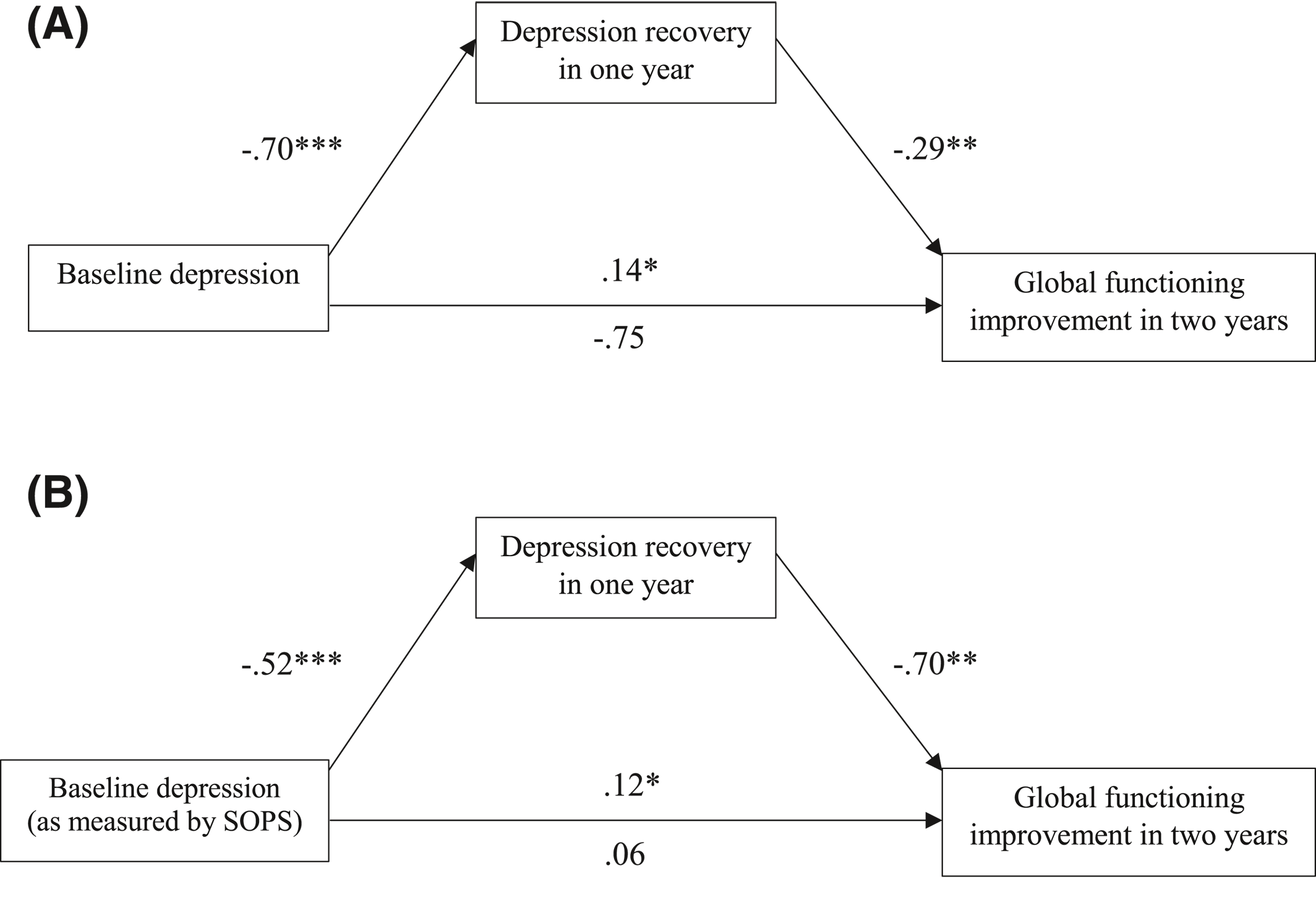
FIGURE 1. Mediation analysis for variables predicting global functioning improvement in 2 years. A. Baseline depression was measured by Calgary Depression Scale for Schizophrenia. B. Baseline depression was measured by The Scale of Psychosis‐Risk Symptoms, depression subscale. β for independent variables and mediators was reported. †p < 0.1. *p < 0.05. **p < 0.01. ***p < 0.001
To test the robustness of the above findings, especially the novel finding on baseline depression as a predictor for functioning improvement in CHR, another model was created in parallel. Keeping all other measures of interest unchanged, the second set of models measured depression with the SOPS depression subscale as defined previously. The results were consistent with the original linear regression predicting functioning outcome at 1 year (R2 = 0.04) and 2 years (R2 = 0.09); specifically, in parallel with the earlier models, higher levels of depression (as measured using the SOPS) were predictive of functional improvement at 12 months (t = 2.75, p = 0.006) and at 24 months (t = 2.92, p = 0.004). The mediation model also yielded similar results; specifically, recovery in depressive symptoms (as measured by the SOPS) at 12 months significantly mediated the relationship between baseline depression and global functioning improvement at 24 months (Figure 1B; 95% CI [0.11, 0.69]). As before, the same mediation was not found in the inverse relationship between baseline positive symptoms and longitudinal functional improvements (95% CI [−0.11, 0.08]).
Predicting the Direction of Global Functioning Change
The same set of baseline symptoms and neurocognitive scores was used in logistic regression models to predict whether global functioning improved (i.e., by any amount) or not at 1 and 2 years (Table 3). Lower levels of positive symptoms (OR = 0.88, p = 0.006) and anxiety (OR = 0.97, p = 0.003), as well as higher levels of negative symptoms (OR = 1.14, p < 0.001) and depression (OR = 1.11, p = 0.022) at baseline predicted global functioning improvement at 2 years, controlling for neutral emotion recognition ability and neurocognitive functioning. These effects remained significant after accounting for the effects of both age and sex.
| Predictors | Dependent variable: global functioning improvement in 1 year | Dependent variable: global functioning improvement in 2 years | ||||
|---|---|---|---|---|---|---|
| B | SE | OR | B | SE | OR | |
| Positive symptoms | −0.05 | 0.03 | 0.96 | −0.13** | 0.05 | 0.88 |
| Negative symptoms | 0.02 | 0.02 | 1.02 | 0.13*** | 0.04 | 1.14 |
| Anxiety | <−0.01† | 0.01 | 1.00 | −0.04** | 0.01 | 0.97 |
| Depression | 0.06 | 0.03 | 1.06 | 0.10* | 0.05 | 1.11 |
| Neurocognitive ability | 0.01 | 0.02 | 1.01 | 0.02 | 0.02 | 1.02 |
| Neutral emotion recognition | −0.10 | 0.12 | 0.90 | 0.15 | 0.15 | 1.16 |
| Positive symptoms | −0.05 | 0.03 | 0.95 | −0.12** | 0.05 | 0.89 |
| Negative symptoms | 0.01 | 0.02 | 1.00 | 0.13** | 0.04 | 1.14 |
| Anxiety | −0.01 | 0.01 | 1.00 | −0.03* | 0.01 | 0.97 |
| Depression (as measured in SOPS) | 0.10** | 0.04 | 1.10 | 0.06 | 0.05 | 1.06 |
| Neurocognitive ability | 0.01 | 0.02 | 1.01 | 0.02 | 0.02 | 1.02 |
| Neutral emotion recognition | −0.09 | 0.12 | 0.91 | 0.16 | 0.16 | 1.17 |
Treatment Issues
As this was a naturalistic study, participants did not receive treatments as a part of their participation, though they did have frequent contact with trained clinical staff as a part of the research assessments. To examine whether depressive symptom recovery or global functioning improvement may be correlated with treatments received in the community, independent samples t‐tests were carried out to compare those who were in therapy or on antidepressants versus those who were not in terms of changes in depressive symptoms and global functioning from baseline to 12 and 24 months, respectively. Both groups (those with vs. without undergoing therapy/antidepressants between the baseline and follow‐up assessment timepoints) showed significant improvement in global functioning and recovery in depressive symptoms from baseline to 12 and 24 months (ps < 0.001), but not differentially with respect to each other.
DISCUSSION
In contrast to the pattern observed among patients with schizophrenia, in which comorbid depression is associated with worse long‐term outcomes, in this study baseline depressive symptoms in the CHR‐P population were found to predict better global functional outcomes. Furthermore, the degree of recovery of depressive symptoms in the first year following baseline completely mediated the association between depressive symptoms at baseline and functional improvement at 2 years. These findings support the view that among those at risk for psychosis, depressive symptoms may signal the presence of an affective subtype, that is, more likely to recover, perhaps because of the episodic nature of depression (31, 32) and/or positive response to treatments.
Our findings on the functional implications of the presence of affective symptoms within the CHR‐P population differ from those among patients with full‐blown schizophrenia. Depression comorbidity may characterize worse outcomes in schizophrenia, as depression may indicate a more serious disorder presentation (9, 33). In particular, depression may manifest as a response to prolonged social isolation and other concomitants of a more serious, fully developed form of schizophrenia. For example, worsening negative symptoms in schizophrenia may reflect a more severe impairment in effort‐cost decision‐making mechanisms. Given that such mechanisms are affected in both depression and schizophrenia, the same impairment can then exacerbate depressive symptoms associated with effort‐cost decision‐making, perhaps manifesting in the form of anhedonia and social withdrawal. In turn, patients with a heightened level of symptoms of both depression and schizophrenia may suffer worse functional impairments, have more limited social relationships, and lower quality of living than those without comorbid depression.
Our findings, together with the theorized model of affective pathway to psychosis, refute the same role of co‐occurring depression in the CHR‐P population. At an early phase of the developmental trajectory of psychosis, co‐occurring depressive symptoms predict better long‐term global functional outcomes. The fact that this relationship was fully mediated by the amount of recovery of depressive symptoms over the first year of follow‐up highlights the value of focusing on depressive symptoms in psychosis as a potential treatment target. In comparison with cognitive impairments and other symptom dimensions in psychosis, co‐occurring depressive symptoms may be more responsive to treatment and more closely associated with better long‐term outcomes (e.g., through improving social functioning and quality of life). In this naturalistic study design, participants were afforded a high degree of contact with trained clinical staff even though treatment per se was not a part of the research protocol. Those who were and were not receiving psychological and/or pharmacological treatments outside of the study protocol did not differ from each other in terms of depression recovery or functional improvement.
The differential roles between depressive symptoms in CHR‐P and comorbid depression in schizophrenia have underlined the potential importance of early identification and intervention for psychosis. The findings of this observational study raise the important possibility that providing early treatments targeting affective symptoms in the CHR‐P population may increase the likelihood for depression recovery and lead to subsequent improvements in long‐term global functional outcomes. This possibility needs to be tested in a randomized controlled study design.
It is worth noting that the amount of recovery in depression did not mediate the inverse relationship between baseline positive symptoms and long‐term functioning within the CHR‐P population. While both depression and positive symptoms are important baseline predictors for global functional outcomes, the two symptom dimensions appear to impact on global functioning through independent pathways. This pattern coheres with the theoretical distinction between the cognitive and affective pathways to psychosis. Our findings further strengthen the claim that, while severe baseline positive symptoms may predict a more chronic subtype of psychosis and worse global functional outcomes, co‐occurring depression at an early stage of psychosis risk characterizes a distinctive subtype, with symptoms potentially stemming more from affective dysregulation than neurocognitive impairments. Given the wealth of evidence‐based treatments for emotion dysregulation and depression (34, 35), the affective subtype of psychosis has a greater potential for recovery through treatments, and could therefore be associated with long‐term functioning improvements.
While baseline negative symptoms and anxiety did not relate to the amount of global functioning changes, the two significantly predicted the direction of changes (i.e., whether global functioning improved or not) at 2 years. Our findings reveal that baseline negative symptoms and anxiety may be less robust predictors of long‐term global functional outcomes than baseline depressive symptoms in that the former are not as sensitive in explaining the degree of recovery over time. Furthermore, similar to baseline depressive symptoms, negative symptoms showed positive associations with global functioning improvement at 2 years. It is worth noting that such associations were driven by the three items in the SOPS that are closely aligned with depressive symptoms—social anhedonia, decreased experience of emotions, and occupational functioning impairments. Baseline anxiety, on the other hand, showed a similar pattern as a positive symptoms in predicting a lower likelihood of global functioning improvement over time. This is in line with the threat anticipation model of psychosis, which theorizes that anxiety may elevate stress sensitivity and prompt paranoid thinking and aberrant salience in early psychosis (36, 37). Future studies can further explore the interaction between positive symptoms and comorbid anxiety, to better tease apart how each contributes to longitudinal functional outcomes.
It is worth noting that this study did not find associations between baseline neurocognitive or emotion recognition ability and global functioning improvement. Baseline affective symptoms predicted longitudinal changes in global functioning above and beyond fundamental neurocognitive abilities. The null findings on neurocognitive ability and emotion recognition further support that the observed functioning improvement may mainly be driven by the affective subtype of psychosis, which is characterized by affective dysregulation rather than neurocognitive impairments.
The study is not without limitations. Differential attrition is always a consideration in longitudinal studies. With an attrition rate comparable to other studies of CHR‐P samples (38, 39), this study observed that those who were evaluated at 12‐month follow‐up had significantly higher global functioning and less severe positive symptoms at baseline than those who were lost to follow‐up. While the differential attrition limits the external validity for models predicting outcomes at 12 months, there were no statistically significant differences in demographic variables and baseline symptom scores between those who were evaluated at 24‐month versus those lost to follow‐up. Thus, differential attrition does not appear likely to explain or limit the generalizability of our findings related to global functional outcomes at 24‐month, at which point the demographic variables and baseline scores of study variables matched between those who were followed versus lost to follow‐up. Further, while the CDSS provides a good gauge of depressive symptoms overall, it may be less sensitive to detect the relation between global functioning changes and particular symptom dimensions within depression, such as anhedonia. Future studies utilizing additional measures (e.g., Beck’s Depression Inventory) may contribute to a more fine‐grained understanding of the specific depressive symptoms’ recovery that are driving the longitudinal functioning improvement in the CHR‐P population.
To conclude, the present findings support the view that depression among those at CHR‐P is indicative of an affective subtype, that is, more likely to recover and experience better long‐term outcomes. This pattern highlights the potential importance of treating co‐occurring depressive symptoms at an early stage of psychosis risk.
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39