Toxoplasmosis and Schizophrenia: A Systematic Review and Meta‐Analysis of Prevalence and Associations and Future Directions
Abstract
Background
A potential link between toxoplasmosis with schizophrenia (SCZ) has been extensively studied over the past 2 decades. Our study was aimed to determine whether, beyond an association, the field is primed for randomized clinical trials of anti‐Toxoplasma prophylaxis in Toxoplasma seropositive patients with SCZ.
Methods
We performed a methodological appraisal of toxoplasmosis‐SCZ association studies, a meta‐analysis, and a compilation of claims and pathophysiologic hypotheses.
Results
We analyzed 66 studies with 11,540 patients with SCZ and 69,491 controls. For patients with SCZ, 54 studies targeted Toxoplasma‐IgG seropositivity, 18 targeted Toxoplasma‐IgG serointensity, and 17 targeted Toxoplasma‐IgM seropositivity. For SCZ‐phenotypes, 26 targeted Toxoplasma‐IgG seropositivity, six targeted Toxoplasma‐IgG serointensity, and three targeted Toxoplasma‐IgM seropositivity. Two‐thirds of these studies reported a positive association. Statistically significant associations with SCZ were reported in 31/54 studies, 11/18 studies, and 3/17 studies. Significant associations with SCZ‐phenotypes were reported in 20/26 studies, 2/6 studies, and 0/3 studies, respectively. Toxoplasma‐IgG seropositivity increased the odds of SCZ (OR = 1.91; 95% CI: 1.61–2.27). Heterogeneity across studies was large (I2 = 80.03%). Adjusted analyses for at least age and socioeconomic status/place of residence were done in 17 studies; temporality was addressed only in 4.
Conclusion
A large number of observational studies revealed a modest to large association between toxoplasmosis and SCZ. Although important methodological biases were identified, further association studies are unlikely to change this association and are not justified. It is time to test this association in randomized double‐blind placebo‐controlled clinical trials of first line anti‐Toxoplasma prophylaxis in Toxoplasma seropositive patients with SCZ.
Highlights
We performed a methodological appraisal of 66 association studies published over the past 2 decades exploring the association between toxoplasmosis and schizophrenia (SCZ) or SCZ phenotypes, a meta‐analysis, and a compilation of claims and proposed pathophysiologic hypotheses.
Two‐thirds of the studies reported a positive association and Toxoplasma‐IgG seropositivity was associated with an increase in the odds of SCZ; however, important methodological limitations were identified.
Further association studies are unlikely to change this association and are not justified.
It is time to test the hypothesis that prevention of intermittent subclinical reactivations of T.gondii in the brain of Toxoplasma seropositive patients with SCZ may have a positive impact in their SCZ‐course in randomized double‐blind placebo‐controlled clinical trial settings using first line anti‐Toxoplasma prophylaxis medications.
Selection of first line primary‐prophylaxis anti‐Toxoplasma medication (such as trimethoprim/sulfamethoxazole), as has been the strategy in other clinical setting for high‐risk immunocompromised patients (e.g., Toxoplasma seropositive transplant patients or patients with acquired immune deficiency syndrome) is critical.
There has been an interest over the past 50 years in the association between chronic latent parasitic infections, such as toxoplasmosis, and mental health diseases (1). Toxoplasma gondii (T.gondii) is an ubiquitous intracellular neurotropic parasite, thought to infect about one third of the entire human population (2, 3). In the brain, T.gondii forms intracellular cysts within neurons, establishing chronic infection (4, 5). Animal models show that T.gondii infected mice exhibited behavioral changes, causing fatal attraction to feline predators (6, 7, 8, 9). Moreover, it is thought that exposure to T.gondii causes significant brain and behavioral anomalies in humans (10). Furthermore, latent Toxoplasma infection in the brain has been associated with widespread brain immune activation, increased blood brain barrier permeability, neural disruption, increased dopamine release in dopaminergic neurons (11) and N‐methyl‐d‐aspartate receptor (NMDA) dysfunction (12, 13). Yet, there is heterogeneity in the results across these studies (14). Toxoplasma infection appears to be a risk factor independent of the known genetic risk factors for schizophrenia (SCZ) (15). The global age‐adjusted SCZ prevalence in 2016 was estimated to be 2.8/1000 persons (16) and globally, SCZ cases rose from 13.1 million in 1990 to 20.9 million in 2016 (16, 17). Treatment with medications that have anti‐toxoplasma activity (TATA) (18), such as haloperidol and valproate, have been reported to be associated with lower rates of certain SCZ phenotypes (12).
The potential link between toxoplasmosis and SCZ has been extensively studied in humans, particularly over the past 2 decades (19, 20, 21, 22, 23, 24, 25). Our study aimed to determine whether, beyond an association, the field is primed for randomized clinical trials (RCTs) of anti‐Toxoplasma prophylaxis in Toxoplasma seropositive patients with SCZ. Towards that end, we performed a methodological appraisal of toxoplasmosis‐schizophrenia association studies. We also performed a quantitative meta‐analysis of this association and created a compilation of claims and pathophysiologic hypotheses. The latest meta‐analysis for the association between toxoplasmosis and SCZ was published in 2015 (19) and since then, several additional studies have been published. Studies in this field have examined different types of toxoplasmosis exposures and/or types of SCZ outcomes. This diversity may introduce both heterogeneity in the results, as well as bias, and it is important to understand the possible methodological limitations of the available evidence. Insights from this systematic assessment were used to discuss future directions in the research agenda in this field.
METHODS
Search strategy
We considered studies included in previous meta‐analyses (19, 21, 24, 25, 26) and performed updated PubMed searches to bring the database up to date (last search February 2021; Figure 1). Our search strategy is listed in Appendix 1. We also perused the reference lists of key review papers for the identification of additional pertinent studies. Articles were screened for eligibility by two independent reviewers (Maria Gianniki, Despina G. Contopoulos‐Ioannidis).
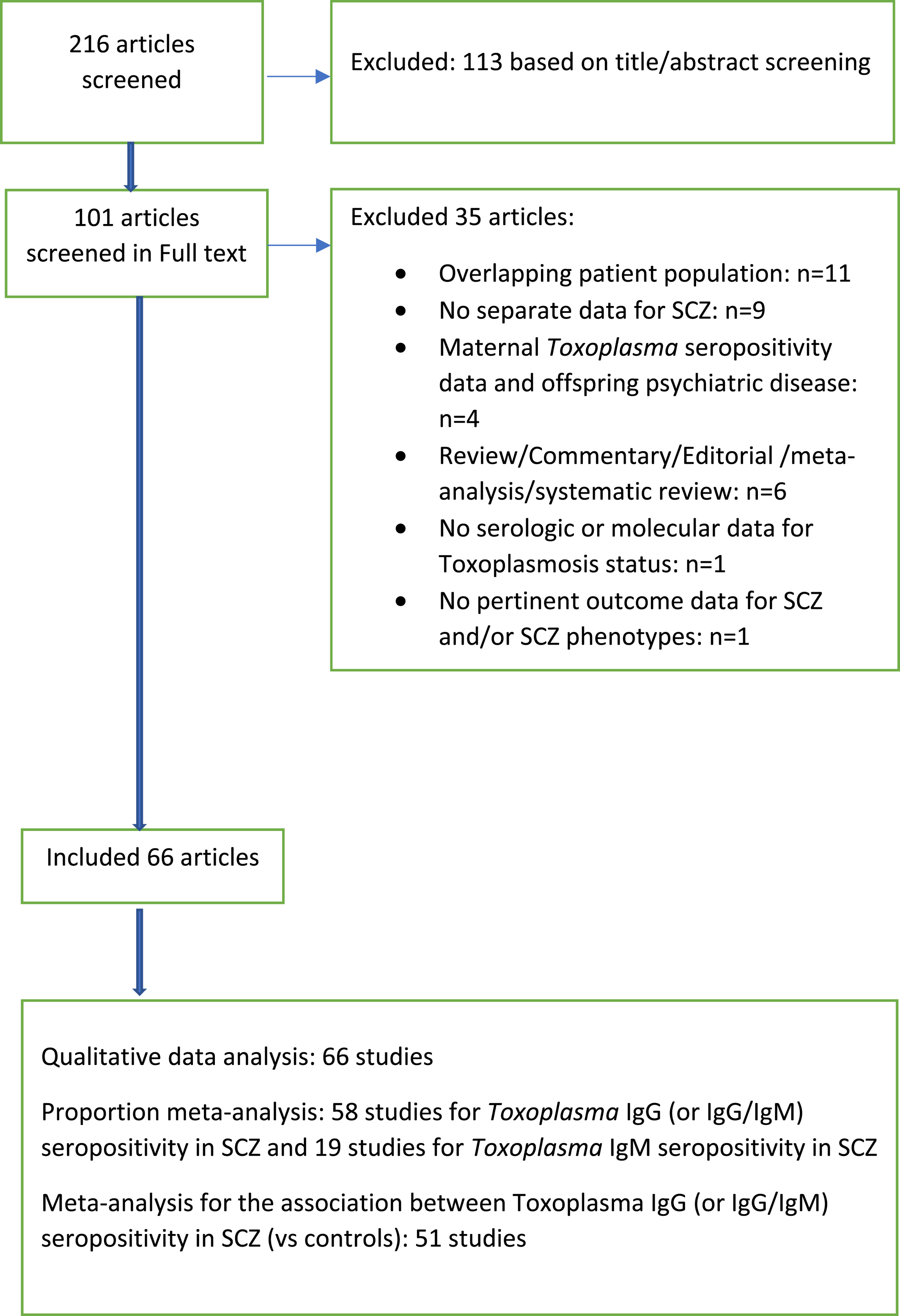
FIGURE 1. Flow chart
Inclusion and exclusion criteria
We included studies of any type of study design (case control studies, SCZ cohort studies and population cohorts) that reported the association between toxoplasmosis and SCZ and/or specific SCZ phenotypes. We kept the definitions for toxoplasmosis and SCZ used by the study authors. The Toxoplasma infection status could have been ascertained by serologic qualitative methods (Toxoplasma IgG or IgM seropositivity), serologic quantitative methods (Toxoplasma IgG or IgM titers/serointensity), or molecular methods. When more than one study existed from the same team with overlapping patients for the same type of SCZ outcome (SCZ or SCZ phenotype), we kept only the publication with the largest number of patients with SCZ. Studies exploring the association between maternal Toxoplasma seropositivity and SCZ in the offspring (27, 28, 29, 30, 31, 32, 33, 34) were not included in our analysis. Positive Toxoplasma IgG antibodies in the newborn infant's Guthrie card blood sample reflect the maternal T. gondii infection status from transplacentally transferred maternal Toxoplasma IgG antibodies to the fetus, and additional neonatal testing is required to confirm whether the newborn infant is congenitally infected or not. We excluded reviews, commentaries and editorials with no original data.
Data extraction
From each eligible study we extracted the following information: author, year of publication, country of patients with SCZ, chronologic period of SCZ patients follow‐up, study design, diagnostic method for ascertainment of toxoplasmosis infection status, type of SCZ outcome targeted (e.g., SCZ and/or specific SCZ phenotypes), study sample size, N of patients with SCZ, N of controls analyzed, N of patients with additional mental health conditions targeted, inclusion criteria for patients with SCZ, types of selected controls (e.g., healthy volunteers, relatives of patients, random sample of patients from other hospital wards, etc.), types of adjustments or matching for confounders between cases and controls, adequacy of adjustments (e.g., adjustments for at least age and socioeconomic status/place of residence), and addressing of temporality (e.g., toxoplasmosis diagnosed before the diagnosis of SCZ).
We considered the following types of toxoplasmosis exposures: Toxoplasma IgG (or IgG/IgM) seropositivity, Toxoplasma IgG serointensity (Toxoplasma IgG titers analyzed as a continuous, categorical or binary variable), Toxoplasma IgM seropositivity, Toxoplasma IgM serointensity (Toxoplasma IgM titers analyzed as a continuous or categorical variable) or Toxoplasma polymerase chain reaction [PCR].
Moreover, we considered the following types of SCZ outcomes: SCZ (any type) or specific SCZ phenotypes (according to age at onset, duration of illness, total SCZ symptom score, positive and negative symptom scores, patient's awareness of illness, patient's compliance with medications, specific SCZ course [e.g., first episode, single episode with remission, single episode with partial remission, episodic without residual symptoms, episodic with residual symptoms, continuous], specific SCZ status [e.g., chronic, partially cured, cured], and types of SCZ [e.g., catatonic, disorganized, paranoid, residual, undifferentiated]).
Data extraction from each study was done by two independent investigators (Maria Gianniki/Angeline Ai‐Nhi Truong and Despina G. Contopoulos‐Ioannidis); discrepancies were reached by consensus.
Qualitative data analysis
We analyzed the prevalence of Toxoplasma IgG (or IgG/IgM combined) seropositivity rate in SCZ and in SCZ phenotypes thereof, the prevalence of Toxoplasma IgM seropositivity in SCZ and SCZ phenotypes thereof, the prevalence of Toxoplasma PCR positivity in SCZ and SCZ phenotypes thereof, and the prevalence of Toxoplasma IgG (or IgG/IgM) seropositivity in controls (if applicable). We analyzed the reported associations (and statistical significance thereof along with 95% confidence intervals [CI]) between toxoplasmosis and SCZ (and/or SCZ phenotypes). Moreover, we created a compilation list of reported claims and pathophysiologic theories to support the biologic plausibility of these associations.
Quantitative data analyses
We used descriptive statistics to analyze the study characteristics. We generated a world‐map of the countries of patients with SCZ.
Quantitative data were synthesized by proportion and association meta‐analyses. Proportion meta‐analyses were also used as not all studies had a control group to allow for the calculation of an association effect size. Proportion meta‐analyses synthesized across studies the Toxoplasma IgG (or IgG/M) and IgM seropositivity rates in patients with SCZ (and in controls respectively) and calculated an average Toxoplasma IgG (or IgG/IgM) and IgM seropositivity rate in these two groups (and 95% CI thereof) across studies. Association meta‐analyses synthesized across studies the effect sizes for the association of toxoplasmosis in SCZ versus controls, and calculated a summary odds ratio (OR) of Toxoplasma IgG (or IgG/IgM) seropositivity in SCZ and 95% CI thereof. When both adjusted and unadjusted effect sizes or raw data were reported, we always preferred the adjusted effect sizes over other metrics. The data across studies were synthesized using the DerSimonian and Laird random effects model (REM) method (35). Studies in these models were weighted by the inverse of their variance (35). The heterogeneity across studies was calculated using the I2 metric (36). I2 > 75% was considered a large heterogeneity. When there is large heterogeneity across studies in a meta‐analysis, the average estimates may be misleading; in those cases, reporting of the median and interquartile range (IQR) of the respective estimates across studies may be more informative. The Egger's test of bias (37) was applied to test for small study effect bias; the Begg's funnel plot was also created.
Predefined subgroup association meta‐analyses were performed according to study adjustment status between SCZ and controls (studies with and without adjustment for important confounders [e.g., age, socioeconomic status/place of residence]), according to the assessment of the temporality of Toxoplasma IgG seropositivity in relation to the time of SCZ diagnosis, and according to study design (population cohorts or case control studies).
We used meta‐regression analyses to analyze across studies the association between the effect size (logarithm of the OR [logOR] of the association between Toxoplasma IgG [or IgG/IgM] seropositivity and SCZ) and the control group Toxoplasma IgG (or IgG/IgM) seroprevalence. The metareg STATA command was used. Moreover, we used multivariate regression analyses to explore the impact of different factors (adjustment for confounders; assessment of temporality, study design or sample size) in the reported effect size of the association. The results of the meta‐analysis were reported according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines (38) (Appendix 2).
Statistical analyses
Analyses were performed in STATA software (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP). The Comprehensive Meta‐analysis software (Comprehensive Meta‐Analysis Version 3 Borenstein, M., Hedges, L., Higgins, J., & Rothstein, H. Biostat, Englewood, NJ 2013) was also used to compile effect size data reported in diverse formats across studies (raw 2 × 2 data, OR, risk ratio [RR] or hazard ratio [HR]).
RESULTS
Study characteristics
We identified 66 studies with non‐overlapping SCZ patient populations published over the past 2 decades (2001–2020), with 11,540 patients with SCZ and 64,491 controls (Appendix 3). Figure 1 shows the flow chart for the identification of these studies. Patients with SCZ were from 24 countries, with top contributing countries being USA (10/66), Iran (10/66), Turkey (7/66), China (4/66), France (4/66), and Germany (4/66; Appendix 4a,b). There were 51 (77.3%) case control studies, four (6.1%) population cohorts, and 11 (16.7%) cohort studies that included only patients with SCZ (Table 1). The median number of patients with SCZ analyzed in these studies was 95 (IQR: 45–180; range: 5–1719). Additional psychiatric patients, except for patients with SCZ, were targeted in 26 (39.4%) studies (Table 1).
| Study characteristics | N (%) of studies (or median N [IQR, range] of patients) |
|---|---|
| Number of studies | 66 (100%) studies |
| Study design | |
| Case control studies | 51 (77.3%) studies |
| Population cohorts | 4 (6.1%) studies |
| Cohorts of only SCZ patients | 11 (16.7%) studies |
| Top countries (for location of SCZ patients) | |
| USA | 10 (15.2%) studies |
| Iran | 10 (15.2%) studies |
| Turkey | 7 (10.6%) studies |
| China | 4 (6.1%) studies |
| France | 4 (6.1%) studies |
| Germany | 4 (6.1%) studies |
| Type of psychiatric patients targeted | |
| Only SCZ patients | 40 (60.6%) studies |
| SCZ along with other psychiatric patients | 26 (39.4%) studies |
| Study sample size | |
| All study patients (median [IQR; range])c | 198 (113–423; 51–45,609) |
| SCZ patients (median [IQR; range]; total N) | 95 (45–180; 5–1719); 11,540 SCZ patients |
| Controls (median [IQR; range]; total N) | 95 (50–214; 20–45,529); 69,491 subjects |
| Types of analyses targeted | |
| N (%) of studies included in the qualitative data analyses | 66 (100%) studies |
| N (%) of studies included in the quantitative data meta‐analyses: | |
| Proportion meta‐analysis of Toxoplasma IgG (or IgG/IgM) seropositivity in SCZ | 58a (87.9%) studies |
| Association meta‐analysis of Toxoplasma IgG (or IgG/IgM) seropositivity in SCZ versus controls | 51 (77.3%) studies |
| N (%) of studies included in the association analyses | |
| Associations with SCZ | |
| Toxoplasma IgG (or IgG/IgM) seropositivity in SCZ (vs. controls)b | 54b (81.8%) studies |
| Toxoplasma IgG serointensity in SCZ (vs. controls) | 18 (27.3%) studies |
| Toxoplasma IgM seropositivity in SCZ (vs. controls) | 17 (25.7%) studies |
| Toxoplasma PCR in SCZ (vs. controls) | 1 (1.5%) study |
| Associations with SCZ phenotypes | |
| Toxoplasma IgG (or IgG/IgM) seropositivity in SCZ phenotypes | 26 (39.4%) studies |
| Toxoplasma IgG serointensity in SCZ phenotypes | 6 (9.0%) studies |
| Toxoplasma IgM seropositivity in SCZ phenotypes (vs. controls) | 3 (4.5%) studies |
| Adjustments in the association effect sizes | |
| No adjustments/matching (between cases and controls) | 49 (74.2%) |
| Adjustment/matching for at least age and socioeconomic status/or place of residence (between cases and controls) | 17 (25.8%) |
| Temporality | |
| Study addressed temporality (toxoplasmosis diagnosed before SCZ diagnosis) | 4 (6%) |
Proportion meta‐analyses
The average Toxoplasma IgG (or IgG/IgM) and IgM seropositivity rates (by REM) among patients with SCZ were 45% (95% CI: 36%–53%; I2 = 99.12%) and 5% (95% CI: 2%–9%; I2 = 91.38%), respectively (Appendix 4c,4e). The respective seropositivity rates among controls were 30% (95% CI: 27%–34%; I2 = 98.59%) and 1% (0%–2%; I2 = 67.33%) (Appendix 4d, f). The respective median and interquartile (IQR) of seroprevalence rates are shown in Table 2.
| Effect size (summary proportion [%] or summary odds ratio [OR]) and 95% confidence intervals thereof (I2) | |
|---|---|
| Proportion meta‐analyses (by random effect models [REM])a | |
| Toxoplasma IgG (or IgG/IgM) seropositivity rate across studiesb,c | |
| Proportion meta‐analysis of IgG (or IgG/IgM) seropositivity rate in SCZ | 45% (36%–53%; I2 = 99.1%) |
| Proportion meta‐analysis of IgG (or IgG/IgM) seropositivity rate in controls | 30% (27%–34%; I2 = 98.6%) |
| Toxoplasma IgM seropositivity across studiesd,e | |
| Proportion meta‐analysis of IgM seropositivity rate in SCZ | 5% (2%–9%; I2 = 91.4%) |
| Proportion meta‐analysis of IgM seropositivity rate in controls | 1% (0%–2%; I2 = 67.3%) |
| Association meta‐analyses (REM)f | |
| Meta‐analysis of Toxoplasma IgG (or IgG/IgM) seropositivity rate in SCZ (vs controls; summary OR by REM) | 1.91 (1.61–2.27; I2 = 80.0%) |
| Subgroup association meta‐analyses | |
| Association meta‐analyses according to adjustment status | |
| Studies with adjustment/matching for age and socioeconomic status/or place of residence (summary OR by REM) | 2.21 (1.63–3.02; I2 = 67.3%) |
| Studies with no such adjustments (summary OR by REM) | 1.79 (1.47–2.19; I2 = 80.8%) |
| Association meta‐analyses according to temporality assessment status | |
| Studies addressing temporality (summary OR by REM) | 1.68 (1.23–2.31; I2 = 0%) |
| Studies not addressing temporality (summary OR by REM) | 1.94 (1.62–2.32; I2 = 81.2%) |
Associations with SCZ
Diverse types of toxoplasmosis exposure and SCZ outcomes metrics were targeted across studies. Some studies targeted more than one type of toxoplasmosis exposure metrics, and more than one type of SCZ outcome metrics. Figure 2 demonstrates the multifarious types of targeted toxoplasmosis exposure‐metrics (Toxoplasma IgG/or IgG/IgM seropositivity, Toxoplasma IgG serointensity‐as a continuous/categorical/or binary variable‐, Toxoplasma IgM seropositivity, Toxoplasma PCR positivity) and SCZ outcome‐metrics (SCZ/or SCZ phenotypes) across studies and the statistical significance thereof of the targeted association‐analyses. Moreover, the number of studies with positive association claims across studies is shown in Appendix 5. Two thirds of the studies (44/66) reported a positive association between at least one targeted type of toxoplasmosis exposure and a SCZ/or SCZ phenotype outcome. A compilation list of all positive and negative claims per individual studies is shown in Appendix 6.
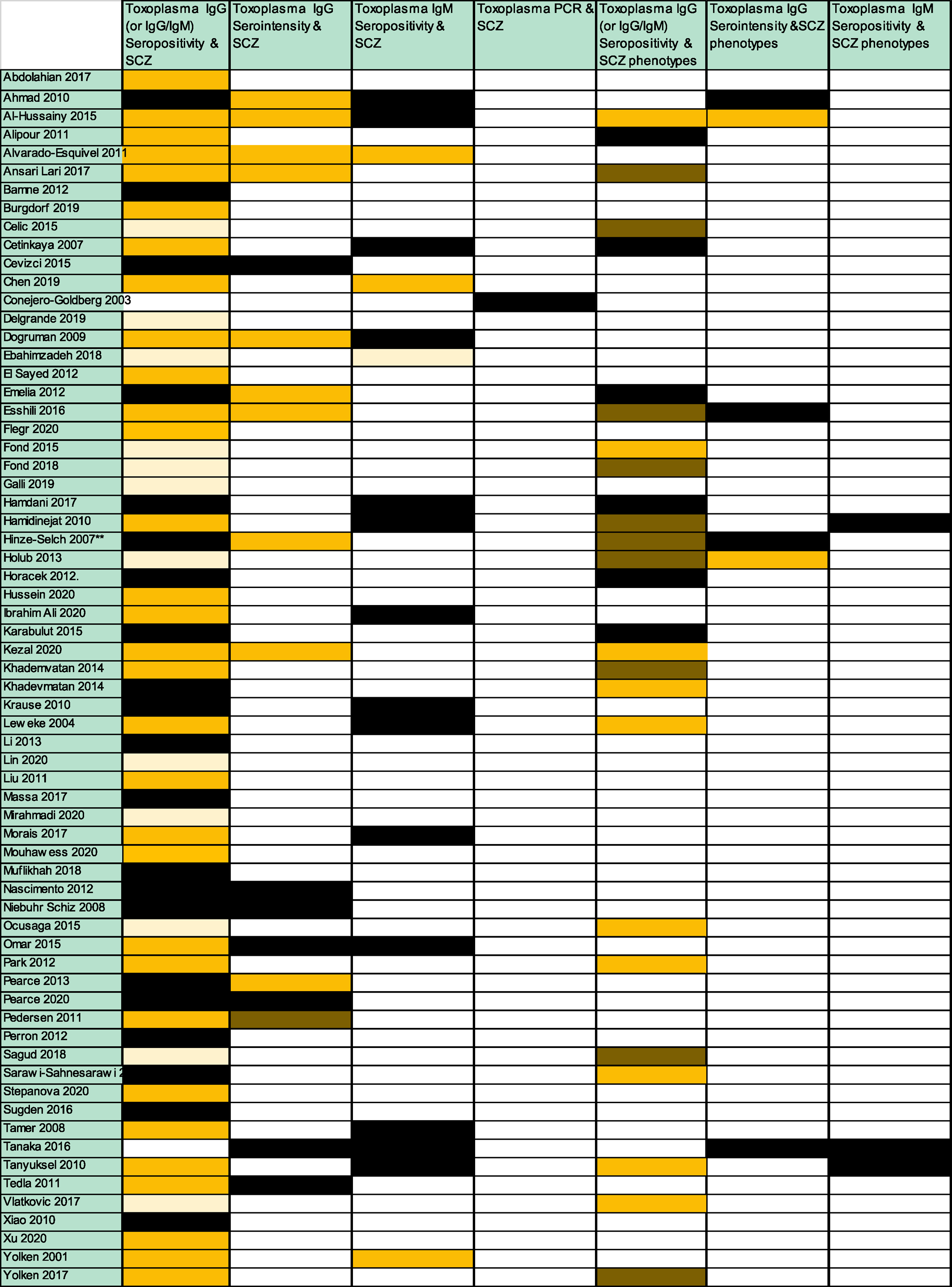
FIGURE 2. Analyzed associations between toxoplasmosis and schizophrenia (SCZ; or SCZ phenotypes). Dark yellow box: indicates studies that tested this association and found statistically significant results. Black box indicates studies that tested this association and found non‐statistically significant results; Dark brown box: indicates studies that tested this association and found both statistically significant and non‐statistically significant results (e.g., statistically significant and non‐statistically significant results according to the type of analysis used for the association between toxoplasmosis and SCZ; or statistically significant and non‐statistically significant results for the association between toxoplasmosis and different types of SCZ phenotypes). Light yellow box: indicates studies that addressed only the prevalence of Toxoplasmosis in SCZ patients (but did not have a healthy control group to assess the statistical significance of the association); White box: indicates studies that did not address this association. Please note that for each association‐category, more than one actual analysis could have been performed per study. For example, a study with dark yellow for the association between Toxoplasma IgG serointensity and SCZ, could have used more than one statistical analysis methods to analyze this association (e.g., the Toxoplasma IgG serointensity could have been analyzed as a binary variable, categorical variable and/or as a continuous variable). Moreover, a study with dark yellow for the association between Toxoplasma IgG seropositivity and SCZ‐phenotypes, could have analyzed more than one type of SCZ phenotypes
The Toxoplasma‐IgG (or IgG/IgM) seropositivity in SCZ versus controls was targeted in 54 studies (81.8%); 51 (77.3%) of those studies provided quantitative data in such a format that could be included in the association meta‐analysis. Eighteen studies (27.3%) targeted Toxoplasma‐IgG serointensity, 17 (25.7%) targeted Toxoplasma‐IgM seropositivity, and 1 (1.5%) targeted Toxoplasma‐PCR (Table 2). Statistically significant associations were reported in 31/54 studies (47.0%) for Toxoplasma IgG/or IgG/IgM seropositivity and SCZ, 11/18 studies (16.7%) for Toxoplasma IgG serointensity and SCZ, 3/17 studies (4.5%) for Toxoplasma IgM seropositivity and SCZ, and 0/1 studies (0%) for Toxoplasma PCR positivity and SCZ, respectively (Figure 2, Appendix 5).
The median number of association‐categories (types of Toxoplasma exposures and SCZ outcomes) targeted per study were 2 (IQR: 1–3; range 1–5; Figure 2); however, the number of actual analyses performed per study was much larger, as several different types of SCZ phenotypes were often targeted for the same type of Toxoplasma exposure (e.g., age of SCZ onset, duration of SCZ symptoms, individual types of SCZ, specific SCZ symptoms scores, etc.). Moreover, the types of toxoplasmosis exposures were often analyzed in more than one way (e.g., Toxoplasma IgG serointensity analyzed as a continuous variable, binary variable and/or categorical variable).
Among studies targeting Toxoplasma IgG serointensity and SCZ, the analyzed associations always pertained to higher mean Toxoplasma IgG titers (or higher number of patients with SCZ in the higher Toxoplasma IgG‐titer percentile thereof), except for 1 study (Kezai et al (39) where SCZ was associated with lower IgG titers. The types of reported Toxoplasma‐IgG serointensity data across studies (e.g., mean Toxoplasma IgG titer, % patients on the top 10th % of Toxoplasma IgG titers, % of patients on the top 25th % of Toxoplasma IgG titers etc.) were too diverse to allow for a meaningful meta‐analysis.
Association meta‐analysis for SCZ
The average OR (by REM) of Toxoplasma‐IgG (or IgG/IgM) seropositivity in patients with SCZ versus controls was 1.91 (95% CI: 1.61–2.27; Figure 3, Table 2). There was large heterogeneity across studies (I2 = 80.03%). The median OR across studies was 1.97 (IQR: 1.24–3.22; Figure 3, Table 2).
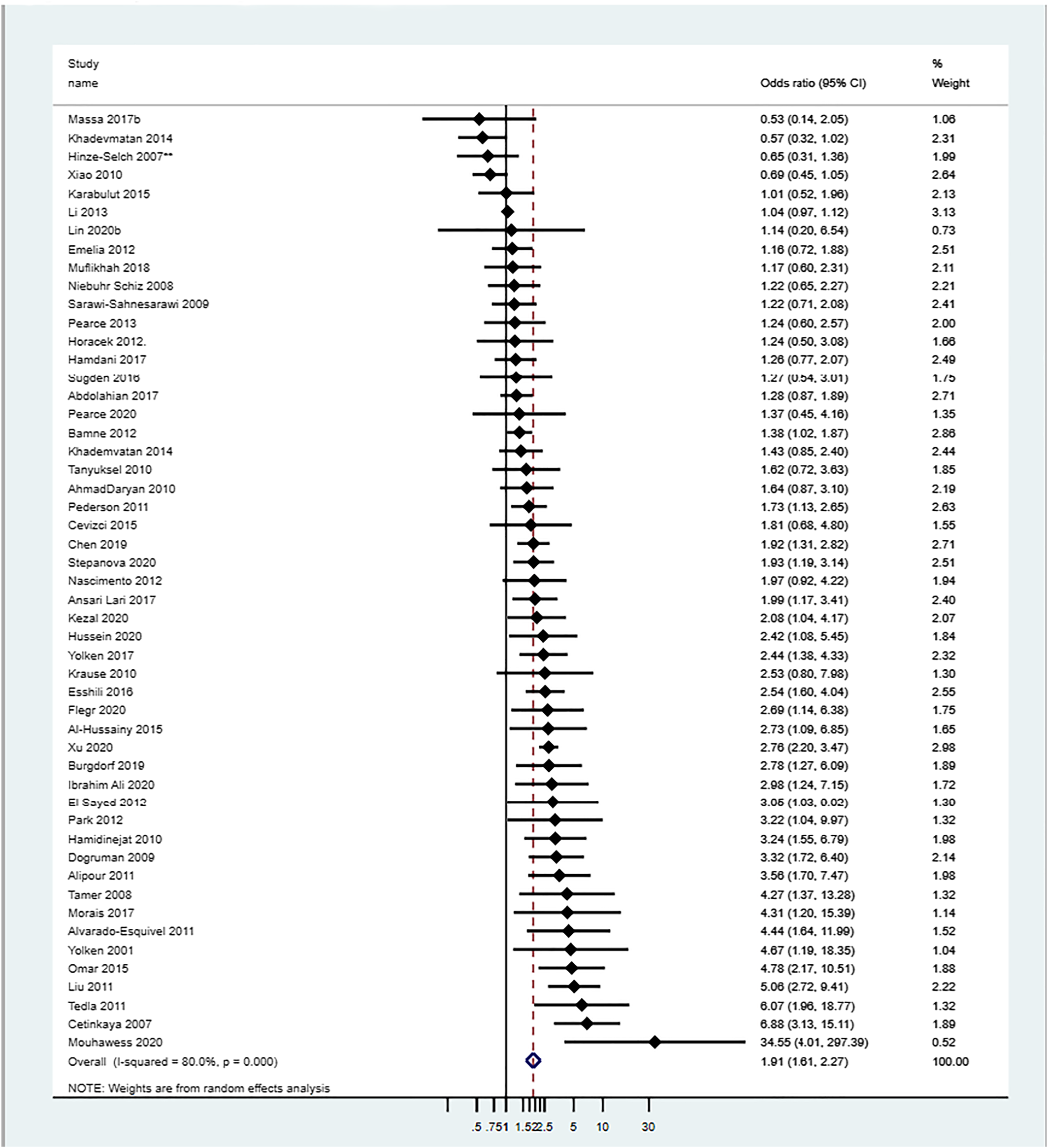
FIGURE 3. Association meta‐analysis: Association of Toxoplasma IgG (or IgG/IgM) seropositivity in schizophrenia (SCZ) patients versus controls. Adjusted OR values were used when provided; ** data were provided only for the subgroup >45 years old. The median OR (interquartile range [IQR]) of Toxoplasma IgG/or IgG/IgM seropositivity in SCZ versus controls was: 1.97 (IQR: 1.24–3.22)
Biases
There was evidence for small‐study effect bias (Egger's test for bias p < 0.001; Appendix 7). Adjusted analyses for at least age and socioeconomic status/place of residence were done only in 17 studies (26%). Moreover, the issue of temporality (diagnosis of toxoplasmosis preceding the diagnosis of SCZ) was addressed only in four studies (6%).
Subgroup meta‐analyses according to adjustment for age/socioeconomic status/place of residence, according to temporality assessment or according to study design, gave similar results (Table 2, Appendix 4g‐i).
Meta‐regression analysis showed a non‐statistically significant downward trend in the association between the effect size (logOR) and the Toxoplasma seropositivity rate in the control group (p = 0.141; Appendix 4j). Moreover, multivariate regression analysis showed no statistically significant association between the OR and the adjustment for age/socioeconomic status/place of residence (p = 0.327), temporality assessment (p = 0.397), study design (p = 0.693), or number of SCZ‐patients analyzed (p = 0.953).
Associations with SCZ phenotypes
The Toxoplasma‐IgG (of IgG/IgM) seropositivity in SCZ phenotypes was targeted in 26 (39.4%) studies; 6 (9.0%) targeted Toxoplasma‐IgG serointensity and 3 (4.5%) targeted Toxoplasma‐IgM seropositivity. Statistically significant associations were reported in 20/26 studies (30.3%), 2/6 (3.0%) studies, and 0/3 (0%) studies, respectively (Figure 2, Appendix 5).
Biological plausibility
Several pathophysiological hypotheses have been proposed for the association between toxoplasmosis and SCZ. Appendix 8 shows a compilation list of these proposed hypotheses as reported in the analyzed studies.
DISCUSSION
We analyzed 66 studies published over the past 2 decades with 11,540 patients with SCZ and 69,491 controls, exploring the association between toxoplasmosis and SCZ or SCZ phenotypes. This large accumulated research agenda reflects the great interest of the scientific community for the identification of potentially preventable and/or treatable risk factors for SCZ (40). Although there was large heterogeneity across studies in the types of toxoplasmosis exposures and SCZ outcomes targeted, on average, 45% of patients with SCZ were Toxoplasma IgG (or IgG/IgM) seropositive versus 30% of controls. Toxoplasma IgG (or IgG/IgM) seropositivity increased the odds of SCZ by 1.91‐fold. This is similar to the estimate from an earlier meta‐analysis by Sutterland et al. in 2015 (OR = 1.81; 95% CI 1.52–2.17) (19).
Most studies targeted only Toxoplasma IgG (or IgG/IgM) seropositivity in SCZ. In contrast, the association with specific SCZ phenotypes was targeted in less than half of the studies. A positive association between Toxoplasma IgG (or IgG/IgM) seropositivity and at least one SCZ phenotype was reported in only a third of the analyzed studies. We did not perform a meta‐analysis for the association between toxoplasmosis and SCZ phenotypes as a meta‐analysis for this association was recently published by Sutterland et al. (25) in 2020. In this meta‐analysis no overall association was seen between Toxoplasma IgG seropositivity and severity of total, positive or negative SCZ symptoms (25). A significant association was only detected in the subgroup of patients with SCZ with a shorter duration of illness (less than 10 years), with Toxoplasma IgG seropositivity being associated with more severe positive symptoms (25).
We identified important methodological biases in the analyzed studies. Approximately 75% of the studies did not perform adjustments for important confounders such as age and socioeconomic status, or place of residence. Moreover, only four studies (41, 42, 43, 44) had addressed whether the Toxoplasma infection preceded the diagnosis of SCZ. In the absence of temporality assessment, causality is uncertain. Toxoplasma infections may be the cause or the result of SCZ (e.g., due to common environmental exposures during hospitalization of patients with SCZ). Three out of 4 studies properly addressing temporality documented a positive association between Toxoplasma infection and SCZ. Burgdorf et al. 2019, a large prospective cohort study in Denmark, showed increased rates of Toxoplasma infection preceding the diagnosis of SCZ (41). Another prospective population cohort study from Denmark by Pedersen et al. of 45,609 women also showed that high Toxoplasma IgG levels before delivery were associated with a significantly increased risk of developing SCZ spectrum disorders subsequently (44). Furthermore, a study among US military personnel in whom Toxoplasma IgG levels were obtained before the diagnosis of SCZ also showed an association between higher Toxoplasma IgG levels and SCZ (43).
Schizophrenia has traditionally been assumed to be largely a genetic condition, with high heritability (45). However, there is concern that the genetic etiology of SCZ might have been overestimated given the inability to detect large genetic effects (45). Schizophrenia may result from a complex interplay between genetic and environmental factors (46). For example, HLA genes of the major histocompatibility complex (MHC) have the strongest genetic predisposition for SCZ in genome‐wide association studies (47). Similarly, MHC genes may affect susceptibility to Toxoplasma infections (48, 49). Certain HLA alleles (e.g., HLA C*04:01 allele) have been shown to decrease susceptibility to T. gondii infection in patients with SCZ but not in controls (49). Interactions between genetic and environmental risk factors merit further investigation (46). Environmental factors may be extremely complex and heterogeneous regarding infections by different strains and timing of infection.
Although most studies studied the role of chronic latent toxoplasmosis (IgG seropositivity) with SCZ, approximately a quarter of the studies also studied the potential association of acute toxoplasmosis (IgM seropositivity) with SCZ. On average, 5% of patients with SCZ were Toxoplasma IgM seropositive, versus 0% of controls. A significant association between Toxoplasma IgM seropositivty and SCZ was shown in less than 5% of the studies. An earlier meta‐analysis by Monroe et al. (21) showed that Toxoplasma IgM seropositivity was associated with an increase in the odds of acute psychosis. However, positive Toxoplasma IgM results with commercially available tests are often false positive and thus, they cannot be used to diagnose acute Toxoplasma infection (50, 51). Positive Toxoplasma IgM results in commercial labs should always be confirmed with additional tests before the diagnosis of acute toxoplasmosis is made. Studies claiming an association between acute Toxoplasma infection and SCZ based only on positive Toxoplasma IgM results, without additional confirmatory testing, should be viewed with caution.
There is speculated biological plausibility for the role of toxoplasmosis in SCZ. Several experimental lines of research addressing parasite‐induced anatomical, histological, and physiological changes have been published; however, there is heterogeneity in reported results (14). During the chronic latent stage of the infection, formation of bradyzoites in the brain could directly alter the dopamine biosynthesis and cause dopaminergic disturbances involved in psychotic disorders (52, 53). The elicited anti‐T. gondii immune responses may cross‐react with host N‐methyl‐D‐aspartate receptors (NMDAR), causing disruption of neural circuits and cognitive deficits (13). Autoantibodies that bind to the NMDARs may underlie glutamate receptor dysfunction and cognitive impairment found in SCZ (54). Latent Toxoplasma infection has been associated with upregulation of the complement C1Q classic immune pathway, which aids in the clearance of the parasite from the central nervous system with subsequent consequences for the connectivity of neighboring cells and synapses, suggested to be involved in SCZ onset (10). Moreover, complement C4 genes have been proposed in gene‐environment interaction studies as potential susceptibility loci for SCZ and infections (including T. gondii infections) (55). Patients with SCZ have increased plasma levels of complement C4 protein activation products, causing increases in blood brain barrier permeability (56). However, there is heterogeneity in the animal studies about the proposed underlying pathophysiologic mechanisms (e.g., alterations in neurotransmitter release, cyst location, and neuroinflammation) for the association of toxoplasmosis with SCZ (14, 17, 57, 58). This heterogeneity may arise from differences in the behavioral assays used, the timing of the behavioral assays, the T. gondii and mice strains utilized, and the route of infection (14). If T. gondii influences human behavior or disease, the effect may depend on the genetic background of the individual and the context of the T. gondii infection (14).
Several neuroleptic antipsychotic and mood stabilizers have been tested for their ability to inhibit replication of T. gondii (59). Among those, the antipsychotic haloperidol and the mood stabilizer valproic acid have been shown to most effectively inhibit T. gondii growth in vitro (60). McFarland et al. (61) and Neville et al. (62) recently reviewed experimental compounds (61) and clinical approved drugs (62) with anti‐Toxoplasma activity. Chorlton et al. (63) identified four published RCTs (64, 65, 66, 67) testing different medications in patients with SCZ. Several important limitations were identified that likely biased the results towards the null; including failure to target specifically Toxoplasma seropositive patients with SCZ (in 2/4 of these trials) and selecting not clinically appropriate medications. The medications tested in those trials included azithromycin (64), trimethoprim (TMP) monotherapy (65), artemisin therapy (66) and artemether therapy (67). These medications are not considered first line anti‐Toxoplasma treatments (64) and most of those are not even considered acceptable anti‐Toxoplasma treatments (65, 66, 67) and have not been used in other clinical settings (e.g., for treatment or prophylaxis of high‐risk Toxoplasma seropositive patients) (68, 69). Four additional small RCTs are currently listed in ClinicalTrials.gov, testing pyrimethamine monotherapy (70), artemisin plus risperidone (71), valproate (72) and L‐tetrahydropalmatine (73) in patients with SCZ. These trials similarly do not use first line medications and are not targeting only Toxoplasma seropositive patents with SCZ.
Further association studies are unlikely to offer more solid evidence at this point. We believe that randomized double‐blind placebo‐controlled clinical trials are urgently needed to test the role of anti‐Toxoplasma primary prophylaxis (with first line anti‐Toxoplasma prophylaxis medications) in Toxoplasma seropositive patients with SCZ. These trials should be ideally simple in design, pragmatic, multicenter, and with few clinically important endpoints. The COVID‐19 era has taught us that only well designed, large, RCTs are able to provide solid clinical evidence in a timely fashion and to change our clinical practices (74, 75).
The hypothesis underlying a study testing a first line anti‐Toxoplasma prophylaxis medication in Toxoplasma seropositive patients with SCZ is that prevention of local intermittent subclinical reactivations of Toxoplasma cysts in the brain of these patients may positively impact their SCZ course. Currently, there are no clinically available medications for the eradication of bradyzoite tissue cysts (the T. gondii tissue form in chronic latent Toxoplasma infection) (76). The goal of such a study in patients with SCZ should be the prevention of intermittent subclinical reactivation rather than eradication of latent T. gondii infection. This has been the strategy for prophylaxis of immunocompromised patients who are Toxoplasma seropositive (69, 77, 78). The proposed mechanism on how anti‐Toxoplasma prophylaxis may help SCZ is that prevention of subclinical reactivations of Toxoplasma cysts may prevent secondary alterations in neurotransmitters' release and/or neuroinflammation and subsequent worsening of SCZ clinical course. A proof‐of‐concept for such prophylaxis study will require at least 1 year of prophylactic drug since reactivations may be periodical and spaced over time.
The preferred first line anti‐Toxoplasma primary prophylaxis regimen is TMP/sulfamethoxazole (TMP/SMX; one double‐strength or one single strength tablet once daily) (69, 77, 78). Long‐term experience exists for the safety and efficacy of TMP/SMX prophylaxis in several high‐risk immunocompromised patients, for example, Toxoplasma seropositive transplant patients (69, 77) or patients with acquired immune deficiency syndrome (78). The majority of these patients tolerate TMP/SMX without toxicities altering the benefit/risk ratio. Primary prophylaxis in Toxoplasma seropositive hematopoietic stem cell transplant patients is routinely recommended for at least 6 months or even longer for certain patients considered significantly immunosuppressed‐requiring prophylaxis‐for more prolonged periods (69, 77). Prolonged secondary prophylaxis with TMP/SMX is also recommended in patients with recurrent toxoplasmic eye disease in vision threatening areas (e.g., for 12–20 months for certain patients) (79, 80) and for Pneumocystis jiroveci prophylaxis in severely immunocompromised patients (e.g., up to 6–12 months for certain transplant patients) (81).
We propose that Toxoplasma IgG seropositive patients with SCZ should be randomized to primary prophylaxis with a first line anti‐Toxoplasma medication such as TMP/SMX versus placebo. The selection of an appropriate first line anti‐Toxoplasma medication, similar to what is routinely used for primary prophylaxis in other clinical settings, is critical. Moreover, the duration of the prophylactic therapy (e.g., at least 1 year) would be an additional key factor to allow for proper assessment of clinically important impact on SCZ course. Multidisciplinary collaboration between psychiatrists, infectious diseases experts, and research methodologists in the design of such a pragmatic clinical trial should be a priority.
CONFLICT OF INTEREST
All authors report no conflict of interest to declare.
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
61
62
63
64
65
66
67
68
69
70
71
72
73
74
75
76
77
78
79
80
81