Preliminary Evidence of Efficacy and Target Engagement of Pramipexole in Anhedonic Depression
Abstract
Objective
To investigate feasibility and target engagement of high‐dose, add‐on pramipexole treatment in anhedonic depression.
Method
In this open‐label pilot study, we included 12 patients with unipolar or bipolar, moderate‐to‐severe depression and with significant anhedonia symptoms. All patients were on a stable dose of one or a combination of antidepressants and/or mood stabilizers and received 10 weeks of adjunctive pramipexole titrated to a maximum dose of 4.5 mg salt/day. All patients were rated with the Dimensional Anhedonia Rating Scale (DARS), the Montgomery Åsberg Depression Rating (MADRS) and the Snaith Hamilton Pleasure Scale (SHAPS). Serum high‐sensitivity C‐reactive protein (hs‐CRP) was analyzed pre‐ and post‐treatment. Eight patients underwent fMRI pre‐ and post‐treatment and a simplified version of the monetary incentive delay task was used to investigate the effect of treatment on striatal activity during reward anticipation.
Results
DARS, MADRS and SHAPS scores all improved significantly over 10 weeks of pramipexole treatment (p<0.01). Mean levels of hs‐CRP decreased significantly over the course of treatment from mean 3.8 mg/L at baseline to 2.6 mg/L at endpoint (p<0.01). There were significant treatment‐associated increases in reward related activity in several brain areas including the right lateral putamen, anterior left caudate, left posterior putamen, right dorsal caudate, left anterior putamen, and the right nucleus accumbens.
Conclusions
This is the first study to suggest efficacy and target engagement of pramipexole in anhedonic depression. Larger randomized controlled trials are needed to confirm or refute these preliminary findings.
Highlights
In this open‐label pilot study, high dose, add‐on pramipexole was a feasible and well‐tolerated treatment for anhedonic depression
Serum high sensitivity C‐reactive protein decreased over the treatment course
Add‐on pramipexole was associated with increased activity in the ventral striatum
Pramipexole is a dopamine receptor agonist with high affinity for the D3 receptor, possibly with additional anti‐inflammatory properties (1, 2). Pramipexole has beneficial effects on symptoms of depression and anhedonia in patients with Parkinson's disease (PD), partly independent of motor symptom improvement (3). Previous studies testing the antidepressant efficacy of pramipexole in non‐PD depression have been promising for both unipolar and bipolar depression (4), but effect sizes, in this unselected and heterogeneous group of depression, have been too small for broader clinical recommendations. In a series of 42 cases with unipolar or bipolar depression, Fawcett et al. described their clinical experience with adjunctive, high dose pramipexole (5). In this treatment‐resistant sample they reported a clinically meaningful response in more than 75% of the patients that persisted over an average follow‐up time of 16 months. Based on their clinical experience and the dopamine agonistic effects of pramipexole, Fawcett et al. suggested that this treatment may be particularly efficacious in a subtype of depression with a symptom profile of anhedonia and lack of motivation. This hypothesis is consistent with the pathophysiology of motivational anhedonia, involving alterations in both dopaminergic neurotransmission and inflammation (6), but no clinical studies to date have tested the efficacy of pramipexole in a subtype of depression with significant anhedonia symptoms. The aims of the current study were to provide preliminary evidence of efficacy and tolerability of high‐dose, add‐on pramipexole in anhedonic depression and to demonstrate target engagement of pramipexole on reward circuitry and inflammation.
METHODS
Patient Recruitment
Twelve patients (mean age = 45, SD = 16; 8 females) with unipolar or bipolar, moderate‐to‐severe, depression were recruited from outpatient clinics in Lund, Sweden. The sample was enriched for significant anhedonia symptoms, enrolling only those patients with a score of <27 on the Dimensional Anhedonia Rating Scale (DARS, inverse scale) (7). This cut‐off was used since a score of 27 on the DARS was the group median in another major depressive disorder (MDD) sample (8). All patients in the current study were on a stable dose of one, or a combination of several, antidepressants and/or mood stabilizers and received 10 weeks of adjunctive pramipexole titrated to a maximum dose of 4.5 mg salt/day (corresponding to 3.15 mg base form). Depot tablets were taken once daily, and patients were instructed to take 0.375 mg salt/day the first week, 0.75 mg salt/day the second week, 1.5 mg salt/day the third week, 2.25 mg salt/day the fourth week, 3 mg salt/day the fifth week, 3.75 mg salt/day the sixth week and finally 4.5 mg salt/day the seventh week and until the end trial. In the event of side effects, dose increase was stopped, and then resumed again after 1 week if side effects were attenuated. Any treatment responder, defined as those who improved ≥50% on the Montgomery Åsberg Depression Rating Scale (MADRS) (9) or scored > 40 on the DARS, continued their current pramipexole dose with no further increases for the remaining study period. Patient characteristics are summarized in Table 1.
| % Female | 67% | |
| Mean age (SD) | 45.2 (15.7) | |
| Mean BMI (SD) | 30.2 (5.2) | |
| % Tobacco users | 42% | |
| Mean Hs‐CRP, ng/L (SD) | Baseline | Week 10 |
| 3.8 (4.7) | 2.6 (3.5) | |
| % SSRI users | 42% | |
| % SNRI users | 50% | |
| % NDRI users | 17% | |
| % Antipsychotics users | 0% | |
| % Mood stabilizers users | 17% | |
| % Previously treated with ECT | 33% | |
| Anxiety disorder co‐morbidity (%) | 50% | |
| Median number of previous antidepressant trials (range) | 5 (2–14) | |
| >50% decrease on MADRS, responder (%) | 33% | |
| >50% decrease on SHAPS, responder (%) | 25% | |
| Mean pramipexole, mg salt/day, at week 10 (SD) | 3.63 mg salt/day (0.70) | |
Patients were screened with Young Mania Rating Scale (YMRS) (10), the Questionnaire for Impulsive‐Compulsive Disorders in Parkinson's Disease (QUIP) (11) and the Problem Gambling Severity Index (PGSI) (12) every second week of treatment.
Patients were rated with the DARS, the MADRS, the Snaith‐Hamilton Pleasure Scale (SHAPS) (13), the Apathy Evaluation Scale (AES) (14), the Fatigue Severity Scale (FSS) (15) and the Insomnia Severity Index (ISI) (16) at baseline and every second week until study completion at week 10.
High‐Sensitivity C‐Reactive Protein (hs‐CRP) Assay
Serum hs‐CRP levels were analyzed pre‐ and post‐treatment according to clinical standard at the Department of Clinical Immunology, Skåne University Hospital, Lund, Sweden. Limit of quantitation for hs‐CRP was 0.60 mg/L.
Functional Magnetic Resonance Imaging (fMRI) Procedures
Eight patients underwent fMRI pre‐ and post‐treatment. To investigate the effect of treatment on striatal activity during reward anticipation we used a simplified version of the monetary incentive delay task (MID) focusing on the appetitive aspects of the task. The task structure was based on previous studies using simplified versions of the incentive delay task (17, 18, 19, 20). During this task the participant is presented with one of two cues that signals either a high reward (€0.5) or a low reward (€0.01). Whether the participant receives the reward or not is contingent on their response time to a subsequent target cue, and the task was programmed to deliver rewards 75%–80% of the time. To examine the effect of treatment on striatal neural activity we used fMRI blood‐oxygen‐level‐dependent (BOLD)‐signal imaging during the reward‐anticipation phase in our region of interest encompassing the striatum. To this end we created contrasts where activity during the low reward condition (controlling for non‐specific activity) was subtracted from the high reward condition both pre‐ and post‐treatment. Similarly, we compared the pre‐contrast to the post‐contrast to examine change in reward‐related striatal activity as an effect of treatment. Hence, the low‐reward contrast is used as the control condition; it has the same trial structure as the high reward condition (controlling for non‐specific activations related to visual stimulation) but since the reward is so low (approx. 0.01€) little reward related activity is expected. Since the purpose of this study is exploratory, we use an uncorrected threshold of p<0.05 for all analyses, only reporting clusters exceeding 80 mm3 (10 voxels) to protect against false positives. More details on the fMRI procedures, including data collection, processing, analyses and results are given in Supplementary Material S1.
Statistics
The Statistical Package for the Social Sciences for Mac (SPSS) was used for statistical calculations, except for fMRI data. Related‐Samples Wilcoxon signed‐rank test was used to investigate changes in symptom severity and hs‐CRP pre/post treatment with pramipexole. A detailed description of the statistical methods used for the fMRI data is given in Supplementary Material.
RESULTS
Pramipexole treatment was generally well‐tolerated. Of those adverse events that were assessed as at least possibly related to pramipexole treatment headache was the most common (n=10) followed by nausea (n=9). Three patients experienced fatigue, two patients reported skin rashes, two patients loss of appetite, two patients experienced vertigo, one patient depressed mood, one patient mild agitation, one patient overeating and one patient heart palpitations. No one developed symptoms of mania. All of these symptoms resolved, usually after modifications in the dose titration schedule as described above. One patient had mild withdrawal symptoms during pramipexole tapering, which prompted slower tapering. All side effects were mild‐moderate and did not result in any dropouts. Mean end‐dose was 3.63 mg salt/day. DARS, SHAPS, and MADRS scores all improved significantly over 10 weeks of add‐on pramipexole treatment (all p<0.01, see Figure 1A).
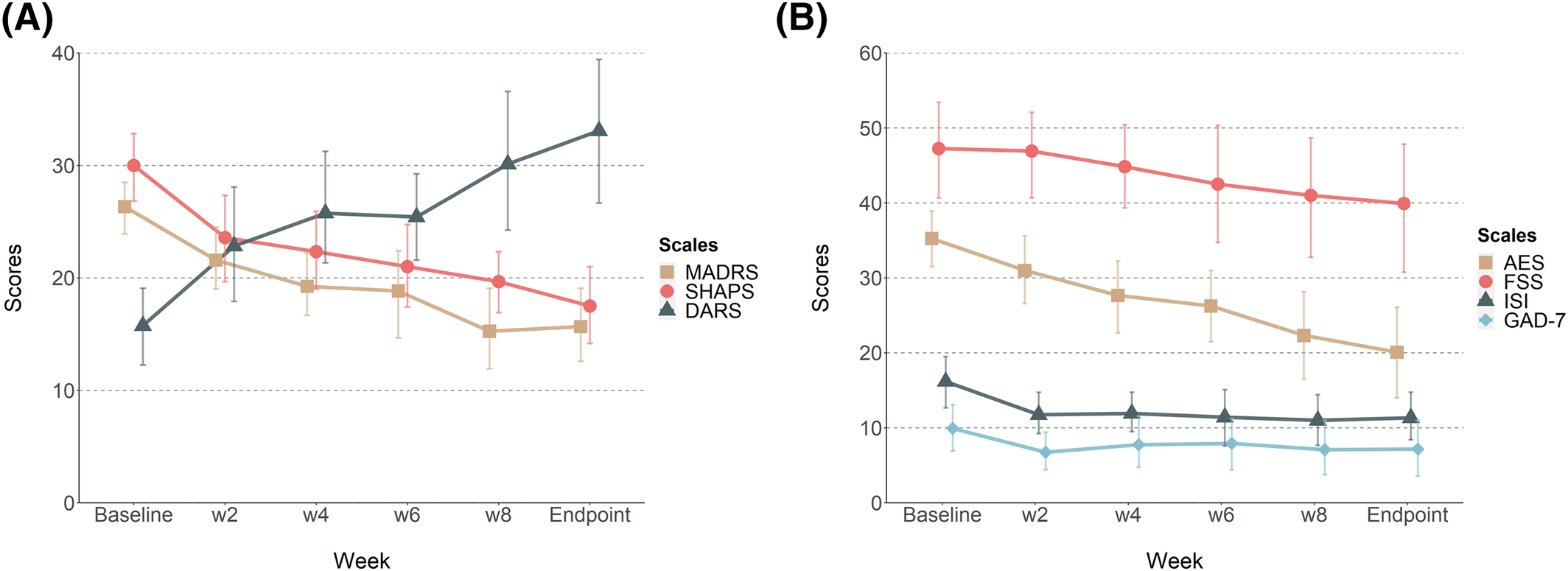
FIGURE 1. Changes in rating scales scores (A) Change in MADRS, SHAPS and DARS scores over treatment (all p<0.01, related‐samples Wilcoxon signed‐ranks test comparing baseline and week 10). (B) Change in AES, FSS, ISI and GAD‐7 scores over treatment. The change from baseline to week 10 in AES was significant (p<0.01) but not the changes in ISI (p=0.054), FSS (p=0.11) or GAD‐7 (p=0.213). Error bars represents 95% CI.
Mean levels of hs‐CRP decreased during the study period from mean 3.8 mg/L at baseline to 2.6 mg/L at endpoint. A Wilcoxon signed‐ranks test indicated that this difference was statistically significant (p<0.01), see Figure 2.
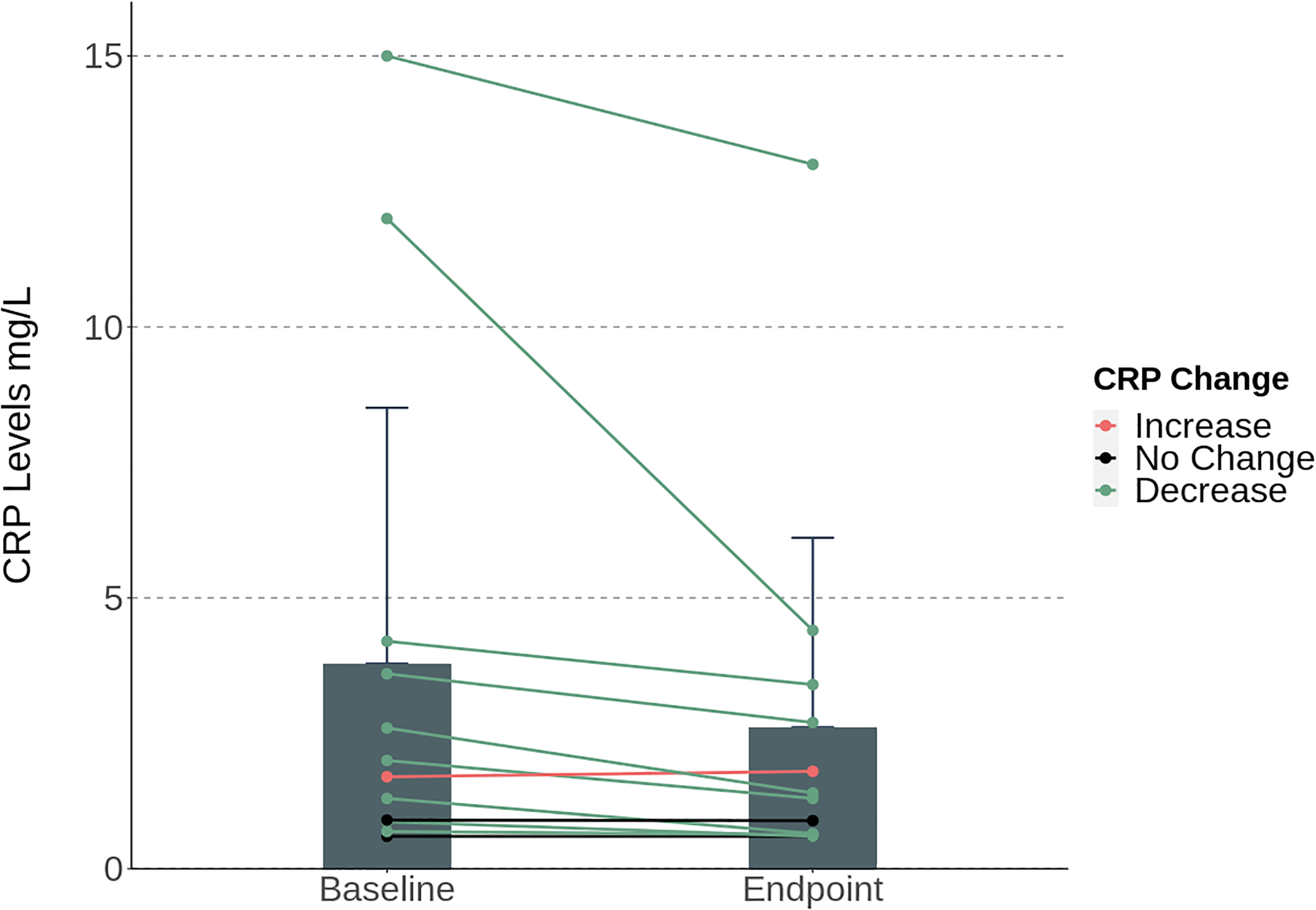
FIGURE 2. Bar graph and individual trend lines of the mean levels of hs‐CRP (mg/L) at baseline versus endpoint (week 10). The mean levels decreased during the study period from mean 3.8 mg/L at baseline to 2.6 mg/L at endpoint. A related‐samples Wilcoxon signed‐ranks test indicated that this difference was statistically significant (p<0.01). Error bars represents ±SEM.
Pre‐treatment, we did not find any indication of significant reward‐related activity during the monetary incentive delay task (MID‐task, fMRI). However, we did observe several regions in the striatum where activity was significantly lower during the reward‐condition (high reward, €0.5) compared to the control condition (negligible reward, €0.01), indicating deactivation. These areas included the left posterior putamen, right dorsal putamen, left dorsal caudate and the right nucleus accumbens (all p<0.01, see Figure 3A). In contrast, post‐treatment we found substantial reward‐related activity in several striatal regions; one small‐cluster in the right ventral caudate extending into the right nucleus accumbens, two clusters encompassing large parts of the left and right lateral putamen and one cluster in the right dorsal caudate (all p<0.01), and indications of deactivation in one small cluster in the medial left caudate (p=0.004). When evaluating pre‐to‐post treatment changes in reward related activity we found significant increases in several areas; a large cluster encompassing the right lateral putamen, anterior left caudate, left posterior putamen, right dorsal caudate (all p<0.01, see Figure 3B), left anterior putamen (p=0.011), and the right nucleus accumbens (p=0.014), and no significant decreases. Since the sample size was small, it was not appropriate to explore correlations between increased striatal activation post‐treatment and treatment outcome of pramipexole. However, the change in striatal activation demonstrates target engagement, and the feasibility of using the MID‐task in larger trials to investigate relationships between treatment outcome and activation of the reward system.
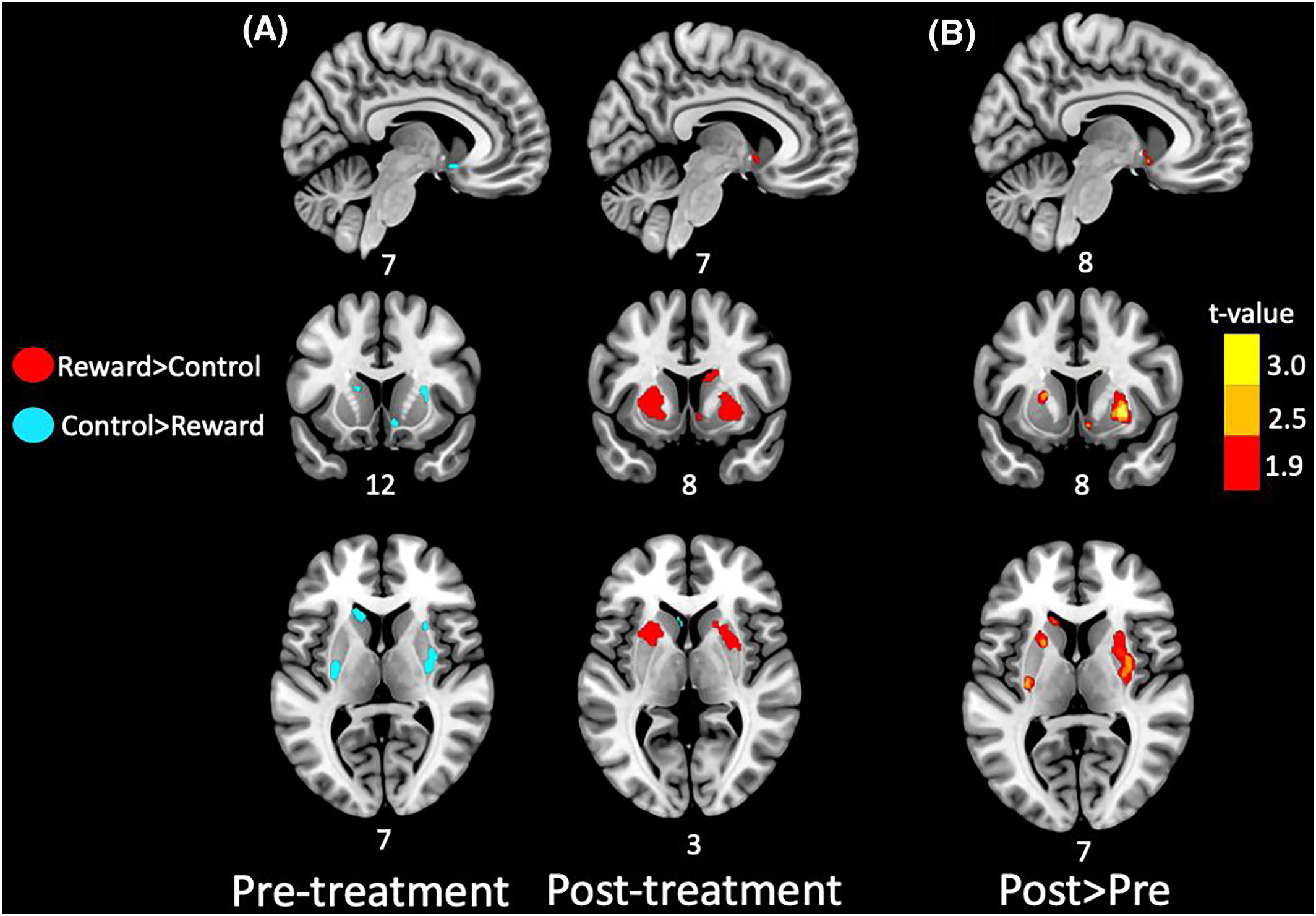
FIGURE 3. Reward‐related striatum activation pre‐ and post‐treatment. (A) Prior to treatment (left panel) we found no evidence of reward‐related activity, but rather indication of deactivation in several striatal regions, including the left posterior putamen (xyz = −30; −10; 8; T = 4.33; p = 0.002; 488 mm3), right dorsal putamen (xyz = 36; −14; −6; T = 4.00; p = 0.003; 1288 mm3), left dorsal caudate (xyz = −14; 20; 10; T = 3.25; p = 0.007; 592 mm3) and right nucleus accumbens (xyz = 8; 8; −12; T = 3.25; p = 0.007; 208 mm3). After treatment (right panel) we found evidence of substantial reward‐related activation encompassing the right nucleus accumbens (xyz = 6; 6; −6; T = 5.06; p = 0.001; 88 mm3), lateral putamen (left: xyz = −18; 10; −2; T = 4.37; p = 0.002; 2912 mm3: right: xyz = 24; 4; −2; T = 5.00; p = 0.001; 3936 mm3), and right dorsal caudate (xyz = 12; 8;−20; T = 3.72; p = 0.004; 1552 mm3). In addition, one small region in the medial left caudate showed deactivation (xyz = −6; 18; 4; T = 3.62; p = 0.004; 112 mm3). Red clusters indicate regions with significant reward‐related activity and blue clusters indicate deactivation, with no color‐coding for strength of activation. (B) When comparing the pre‐treatment to the post‐treatment contrast we found significant increases in reward‐related activity in several regions including right lateral putamen (xyz; 32; −4; 12; T = 4.15; p = 0.002; 3848 mm3), anterior left caudate (xyz = −14; 22; 10; T = 4.07; p = 0.002; 416 mm3), left posterior putamen (xyz = −28; −14; 10; T = 3.99; p = 0.003; 392 mm3), right dorsal caudate (xyz = 20; −2; 24; T = 3.30; p = 0.007; 104 mm3), left anterior putamen (xyz = −20; 10; 8; T = 2.95; p = 0.011; 512 mm3), right nucleus accumbens (8; 8; −10; T = 2.74; p = 0.014; 224 mm3), and no significant decreases. Clusters are color‐coded for strength of activation increases. Only voxels passing an uncorrected threshold of p<0.05 with at minimum cluster extent of 80 mm3 are shown. Numbers adjacent to brain images indicate MNI‐coordinates of the slice. Results are displayed on an MNI‐template.
DISCUSSION
This is the first study to demonstrate preliminary efficacy, tolerability and target engagement of pramipexole in anhedonic depression. Our findings provide an important new lead in the pursuit of personalized psychiatry; specifically, they raise the possibility that dopamine agonist pramipexole is a useful intervention for a subgroup of depressed patients with an anhedonic symptom profile. Moreover, this is the first clinical study to demonstrate an increase in reward circuitry activity and a decrease in peripheral inflammatory markers following pramipexole treatment. These findings are consistent with the well‐known dopamine agonistic effects of pramipexole, and with one previous study showing that pramipexole reduces anhedonia in an animal model of inflammatory depression paralleled by a decrease in inflammatory markers in the hippocampus (2).
We found that pramipexole treatment was associated with increased activity in the ventral striatum, per the MID task. The MID elicits an increase in BOLD activity in the ventral striatum during reward anticipation in healthy controls (17), while this response is weaker in depressed individuals (21). Consistent with a clinically meaningful subtype of anhedonic depression, a recent large‐scale study showed that resting state connectivity (assessed by fMRI) was able to delineate different “biotypes” of depression predicting treatment response to transcranial magnetic stimulation therapy (22). Interestingly, anhedonia‐related symptoms were more common in certain biotypes characterized by dysfunctional connectivity in brain networks associated with reward processing. Despite all the limitations and caveats inherent to the small‐scale, open label design of the current study, our results suggest that pramipexole targets the reward circuitry in depression, consistent with a specific effect on anhedonia symptoms, and future large‐scale, placebo‐controlled trials should aim to replicate these findings before any clinical implications can be made.
Our findings are consistent with several lines of experimental data and clinical observations suggesting that both dopaminergic and immune‐related alterations map onto a subtype of anhedonic depression (6, 23). Inflammatory cytokines have an impact on mesolimbic dopamine signaling, which can lead to symptoms of motivational anhedonia and changes in reward‐seeking behavior (6). In support of this, an fMRI‐study of depressed patients reported a correlation between hs‐CRP and anhedonia mediated via decreased resting‐state functional connectivity in the reward circuits (24). In further support of a link between inflammation, dopamine and anhedonia, various types of immune challenges (to healthy volunteers, patients with hepatitis or animals) trigger motivational anhedonia (25), a blunted response to reward anticipation in the ventral striatum (26), and decreased striatal dopamine release and availability (25, 27). The molecular mechanisms by which inflammation may lead to a hypodopaminergic state are not fully understood but may involve inflammation‐induced oxidation of enzyme co‐factor tetrahydrobiopterin (BH4) (28), which is involved in dopamine synthesis.
In summary, the results from this pilot study support an anhedonic subtype of depression, characterized by alterations in dopaminergic and inflammatory pathways, that could be specifically targeted with pramipexole, but future RCTs are needed to confirm or refute this hypothesis.
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28