The Use of Holographic Memory Resolution® to Improve the Physical and Biopsychosocial Symptoms of Chronic Pain: A Feasibility, Mixed Methods Study
Abstract
Objective
Holographic Memory Resolution® (HMR®), a mind‐based therapy, has been used for decades as a nonpharmacologic intervention for trauma imprinting to alleviate depression, anxiety, pain, and post‐traumatic stress disorder (PTSD). No clinical studies were found examining the use of HMR®. This study examined the feasibility and preliminary efficacy of administering HMR® to individuals experiencing chronic pain and related biopsychosocial symptoms.
Methods
A feasibility, mixed‐methods study was conducted between October 2021 and July 2022 and included four HMR® sessions over 1–12 weeks. A convenience sample was comprised of 60 adults suffering from chronic physical or emotional pain of 4+ (0–10 scale) over 6+ months at two clinics in the U.S. Baseline and subsequent surveys after sessions 2, 3, and 4 assessed symptom response. Symptoms were longitudinally measured via self‐report of depression, anxiety, somatic symptom burden, PTSD, and vitality.
Results
73% completed all four sessions, demonstrating feasibility. Ages ranged from 19 to 80 years, 85% were female, and 87% were Caucasian. 52% reported high risk for toxic stress. Four symptoms decreased significantly: depression (p = 0.05), anxiety (p = 0.03), symptom burden (p < 0.01) and PTSD symptoms (p = 0.01); vitality improved.
Conclusions
HMR® may be a feasible intervention to address chronic pain and accompanying biopsychosocial symptoms; a randomized controlled trial is the next step to measure efficacy. Unlike other mind‐based therapies, HMR® participants use their own internal language for identification and resolution of the pain. The trauma imprinting can then be gently addressed, and the memory‐based components of pain resolved or reduced, which empowers participants to improve their well‐being.
Trial registration
ClinicalTrials.gov Identifier: NCT05001399.
HIGHLIGHTS
While used for decades, this is the first trial reported in the scientific literature to examine the use of Holographic Memory Resolution® (HMR®) in any population.
HMR® is a feasible intervention to address chronic pain and accompanying biopsychosocial symptoms.
HMR® was found to significantly decrease depression, anxiety, symptom burden, and post‐traumatic stress disorder symptoms in patients experiencing chronic pain.
Chronic pain is a significant health problem and one of the most common reasons individuals seek medical care. Approximately 20.4% (50 million) U.S. adults suffer from chronic pain; 8% consider pain to have high‐impact on their quality‐of‐life (1). Persistent chronic pain is associated with significant emotional distress and functional disability (2).
The updated International Association for the Study of Pain definition recognizes pain within a biopsychosocial framework (3) and considers biological, psychological, and social factors contributing to the experience. A recognizable cause of pain cannot always be found, rather, underlying emotional and social factors are at play generating or triggering the pain and unexplained somatic symptoms (2, 4). Triggers include anxiety, depression, and post‐traumatic stress disorder (PTSD). Emerging studies consider the impact of adverse childhood experiences (ACEs), which include abuse and neglect during childhood years, as notable generators for chronic pain in later life (5).
Plentiful options exist for the management of chronic pain, but effective and safe strategies are lacking. Opioids are an option, but side effects and potential for substance use disorder are major concerns (6). Furthermore, treating physical pain alone is rarely effective due to underlying emotional and social pain generators (7). Therefore, mind‐body therapies (MBTs) such as meditation, cognitive behavioral therapy (CBT), and mindfulness are sometimes employed (8). While the literature is replete with studies testing MBTs, only small reductions in pain are reported (8, 9, 10, 11). A 2020 systematic review from the Agency for Healthcare Research and Quality (AHRQ) assessed the impact of MBTs for chronic pain conditions and suggested more studies in this area (12).
Holographic Memory Resolution® (HMR), a mind‐based therapy without somatic movement was developed by Brent Baum in the early 1990s. He and several others have been using HMR® to treat individuals with a variety of complaints including depression, anxiety, pain, and PTSD. HMR® incorporates elements of energy psychology, guided imagery, and clean‐language interviewing (patient‐centered without practitioner input) into a single approach with the aim of changing the emotional component of a negative memory to resolve psychological distress (13). Despite being used for decades, only unpublished data exist; no published studies were found that report the outcomes of HMR®.
Due to the dearth of research in this area, the primary aim of this study was to examine the feasibility of administering HMR® to individuals experiencing chronic pain and its related biopsychosocial symptoms. Our secondary aim was to measure preliminary efficacy of HMR® on pain and its accompanying symptoms. We hypothesized that HMR® would be feasible and improve anxiety, depression, PTSD symptoms, and vitality in participants with chronic pain. Participant perceptions and self‐determination theory mediators and outcomes were also explored but will be reported elsewhere.
METHODS
Design and Setting
This feasibility, mixed‐methods study was conducted from October 2021 to July 2022. Participants were enrolled at two U.S. locations, a rural Northwest multispecialty clinic and a metropolitan Southwest clinic. The study was approved by a central Institutional Review Board.
Sample
Eligible individuals were 18 years of age or older and suffering from pain for at least 6 months of ≥4 average intensity on a 0–10 scale with “0” being no pain and “10” being worst possible pain. Participants expressed a variety of pain sources including headaches, stomach pain, back pain, and other musculoskeletal pain. Accompanying symptoms included anxiety, depression, stomach and bowel issues, emotional trauma, and pandemic burnout.
Exclusion criteria included those with thought disorders or suicidal ideation. Because the intervention required participants to enter alpha/theta state, individuals taking mood altering substances were excluded. Examples include cannabis, barbiturates, benzodiazepines, opioids, and lithium. This exclusion is based on evidence that alteration of delta, theta, alpha, and beta brain waves occur in patients taking these substances, making it difficult for patients to enter alpha/theta state which is required for HMR (14). Participants were asked to abstain from alcohol at least 48 h prior to session one and during the trial duration.
Procedures
Participant recruitment
Participants were recruited through community flyers and referrals (professional, patient‐patient, and self‐referred). The study was advertised on internal employee announcement boards and posted on ClinicalTrials.gov. Flyers were also mailed to participants with recent encounters at the study sites with a diagnostic code matching study eligibility.
Each site study coordinator, trained in the ethics of human subjects’ research, confirmed eligibility, discussed the study with each prospective participant, and obtained written informed consent. Upon consent, each participant completed baseline measures on paper or via an electronic device. The study coordinator then escorted the participant to the HMR® treatment room, a calm and peaceful space with limited distractions. The certified HMR® practitioner answered any remaining questions.
Intervention
HMR® was comprised of four 90‐min sessions, designed by the following timeline: Session 1 (baseline upon enrollment), Session 2 (1–7 days following baseline), Session 3 (14–24 days after baseline) and Session 4 (8–9 weeks after baseline). Session content is described in Table 1.
| Session | Description of session |
|---|---|
| 1 | Relaxation techniques are introduced, and each participant is invited to close his/her eyes which places the individual in alpha/theta EEG state. They are then asked to identify and describe a safe place or create an imaginary safe scene. The participant is then invited to identify any associated sensory features (ASF) (i.e., olfactory, tactile, gustatory, auditory, visual) or color(s) associated with their emotion(s) experienced in the safe place. Breathwork and the use of ASF are incorporated to diminish distraction, negative intrusion, negative self‐talk, and disruptive thoughts, which could potentially invade their safe place. HMR® processing of memories continues if time allows (see Session 2 as below). Participants are then invited to practice accessing their safe scene several times a day but not while driving or operating equipment. Permission was obtained from each participant at the end of each session to continue with the next session |
| 2 | The practitioner asks the participant what life events occurred since last session and when ready to proceed, are invited to re‐engage in relaxation and safe scene techniques. If the participant has difficulty with accessing their safe scene using color or ASF, these techniques are revisited. The practitioner then asks if the participant is ready to continue. The participant is next asked to metaphorically identify the presence of any pain, pressure, tightness, or tension and locate this tension in the body. This is achieved by guiding the participant through a series of nine questions describing the metaphor |
| 1. When you feel this pain/issue, where do you feel this in your physical body? | |
| 2. Is it on the inside, outside, or both? | |
| 3. Size? | |
| 4. Shape? | |
| 5. Color? | |
| 6. Temperature? | |
| 7. Texture? | |
| 8. Weight? | |
| 9. Anything else? | |
| Each metaphor is individualized according to the participant's responses and often tied to a traumatic experience. The participant is encouraged to describe what occurred during the event. Once the participant identifies the historical moment of traumatic encoding and dysphoric feelings generated at the time of the event, they are given the option to continue or stop review of the memory. The participant then identifies the experience that caused the pain or dysphoric memory or issue and is asked to image or integrate a creative solution to help diminish the dysphoric pain/emotions. The participant is encouraged to utilize a color or other ASF from the resolved image and to breathe or transmit this solution to the body‐mind to help diminish the dysphoric symptoms. Utilization of these ASFs fosters comfort and safety while dysphoric symptoms continue to be identified and released, keeping in mind, historical data is not changed but rather the goal is to ameliorate the dysphoric pain/emotions. Once the participant identifies the creative solution and integrates it internally, the practitioner inquires whether the relevant memory‐based pain is diminished or discharged. Next, the practitioner offers various options for mindfully disposing of the dysphoric memories (e.g., blowing them up, vaporizing them) and continues this emotional reframing with the same or additional memories or to move towards closure, grounding, and anchoring to re‐enter them back into the world. To do so, participants are asked to open their eyes, state where they are, describe their surroundings, and acknowledge the work accomplished during the session. The participant is encouraged to continue utilizing their personal colors or other ASFs to maintain safety and fully anchor the integrated solutions at the close of the session | |
| 3 | This session is conducted similarly to Session 2, using the colors, pain, and safe scene. As the participant recognizes they are safe and better understands the use of HMR with consecutive sessions, they are then enabled to explore deeper levels as they personally choose |
| 4 | Conducted similarly using the colors, pain, and safe scene as described in Session 3 |
Various instruments were used to examine feasibility and detect a signal for change. Self‐reported demographic characteristics (age, sex at birth, race, ethnicity, and marital status) and ACEs were collected at baseline. Symptom measures described below were completed at baseline, prior to Sessions 3 and 4, and approximately 2–4 weeks following Session 4 either on paper/electronic device or via an emailed link. All measures were self‐report. Participants received $10 for each completed survey; those who completed all surveys were provided an additional $10 gift card for their time.
Instruments
Adverse Childhood Experiences Questionnaire (ACE)
The ACE questionnaire is a 10‐item survey with “yes” or “no” questions about exposure to ACE including: (1) types of abuse; (2) types of neglect; and (3) types of family dysfunction. The ACE score indicates cumulative stress during childhood; a score of 1–3 (accompanied by health comorbidities) or ≥4 (with or without any comorbidities) indicates high‐risk for a toxic stress response (15). Internal consistency is well‐established (α = 0.88) (16).
Patient Health Questionnaire (PHQ‐9)
The PHQ‐9 is a 9‐item instrument used to screen, diagnose, and measure depression (17). The suicide intent question was omitted, as the survey could be taken at home without immediate access to mental health services. Items are scored on a 4‐point Likert scale from 0 = not at all to 3 = nearly every day (range 0–27). A meta‐analysis of 58 studies indicated a cut‐off score ≥10 provides 85% specificity for major depression (18). Internal consistency for treatment and follow‐up is α = 0.74 and 0.81 respectively (19). The 8‐item tool has also been shown to be valid and reliable (20).
Generalized Anxiety Disorder‐7 (GAD‐7)
The GAD‐7 is an instrument used to measure anxiety disorder. Items are scored on a 4‐point Likert scale from 0 = not at all to 3 = nearly every day (range 0–21). Mild anxiety is indicated with a score of 5, moderate anxiety 10, and severe 15. A score ≥10 is indicative of a generalized anxiety disorder. Internal consistency is excellent (α = 0.92) (21).
Somatic Symptom Scale (SSS‐8)
The SSS‐8 is an 8‐item instrument used to measure somatic symptom burden. Respondents are asked to rate bother of various types of pain, fatigue, gastrointestinal, and cardiopulmonary complaints on a 5‐point Likert scale (0 = Not at all, 1 = A little bit, 2 = Somewhat, 3 = Quite a bit, 4 = Very much). Scoring ranges from 0 to 32 (no to minimal [0–3 points], low [4–7 points], medium [8–11 points], high [12–15 points], and very high [16–32 points]). The scale has good reliability (α = 0.81) (22).
PTSD Checklist for DSM‐5 (PCL‐5)
The PCL‐5 is a 20‐item questionnaire used to assess the presence and severity of PTSD symptoms (23). This self‐report tool serves to screen, diagnose, and monitor PTSD symptoms over time. Respondents rate how much they have been bothered by each of 20 stressful experiences on a 5‐point Likert scale ranging from 0 to 4 (0 = Not at all, 1 = A little bit, 2 = Moderately, 3 = Quite a bit, 4 = Extremely). Items are summed for a total severity score ranging from 0 to 80 points. A reduction of 5 points suggests a reliable reduction of symptoms; a reduction of 10–20 points reflects clinically significant change (24).
Subjective Vitality Scale—State Level Version (SVS)
The SVS is a 6‐item instrument used to measure the state of feeling alive and alert and is negatively correlated to physical pain (25). Respondents are asked to indicate which 6 positively‐worded statements are true for them on a 7‐point Likert scale, (1 = not true at all, 4 = somewhat true, 7 = very true). Internal consistency is very good (α = 0.80–0.89) (26).
Statistical Analysis
Descriptive statistics were used to describe the study sample. Feasibility measures included attrition, attendance, and completion of measures. Attrition rate was calculated as the number of participants who dropped out of the study divided by the total number of participants enrolled. Feasibility was measured by completion of the intervention and surveys of at least 70% (27). Analyses employed an intent‐to‐treat approach. General linear mixed models were used to test a signal in health outcomes across HMR® sessions (28, 29). All models controlled for age and employed sex as a fixed effect. Associations of participants’ first, last, and percent changes between health outcomes were assessed with the Pearson Correlation Matrix. Analyses were conducted using R statistical software version 4.2.2 and SPSS version 26. Since this study’s primary aim was to establish feasibility of administering the HMR® intervention, statistical power was not considered.
RESULTS
Sample
A total of 60 participants enrolled in the study; the majority were female (85%), Caucasian (87%), and partnered (55%). Participants represented a wide range of ages, 19–80 years with a mean of approximately 50 years. Over half (52%) reported an ACE score at high‐risk for toxic stress. Table 2 provides a full description of study participants.
| Demographic characteristics | |
|---|---|
| Mean ± S.E. age, years | 49.9 ± 1.94 |
| Female, no. (%) | 51 (85) |
| Race, no. (%) | |
| Caucasian | 52 (87) |
| Black | 1 (2) |
| Asian | 1 (2) |
| Native American | 3 (5) |
| Other | 2 (3) |
| Hispanic or Latino, no. (%) | 4 (7) |
| Marital status, no. (%) | |
| Married or partnered | 33 (55) |
| Single | 14 (23) |
| Divorced | 10 (17) |
| Widowed | 3 (5) |
| Health characteristics | |
| Mean ± S.E. ACEs, score | 4.0 ± 0.30 |
| Symptoms began during COVID‐19 pandemic no. (%) | 5 (8) |
| Symptoms worsened during COVID‐19 pandemic, no. (%) | 24 (40) |
Feasibility
Forty‐eight participants (80%) completed three of four sessions of the HMR® intervention (Figure 1). Sixteen participants withdrew from the study: three were lost to follow‐up, and 13 dropped out due to extraneous circumstances (declined to participate, screening failure, travel or time constraints, family emergency, adverse events, death unrelated to the study). Among study completers, 33 (75%) completed all four surveys.
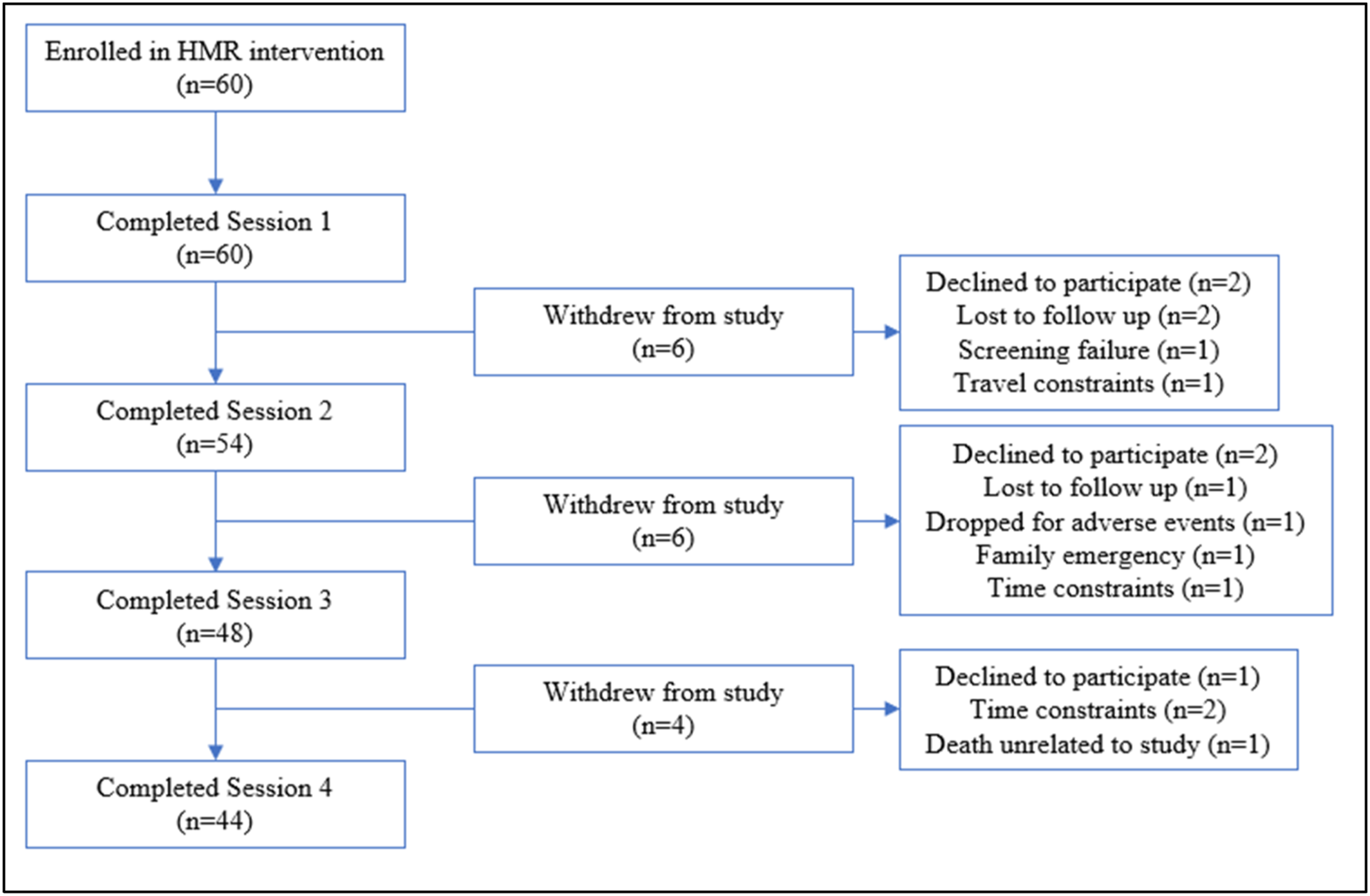
FIGURE 1. Holographic Memory Resolution consort flow diagram.
Most participants completed the intervention outside the specified study protocol timeline due to availability among participants. Namely, several completed their first and second session within 1 week; the third and fourth sessions varied. Most completed their second session within 3 weeks of their first. Seven participants received a rapid, condensed intervention over one to 2 weeks due to living out of state from the study site or having scheduling issues.
Symptom Outcomes
Prior to study implementation, approximately half of participants reported at least moderate levels of depression (n = 33, 55%), anxiety (n = 29, 48%), somatic symptom burden (n = 55, 93%), and PTSD symptoms (n = 30, 50%). Figure 2 provides estimated marginal means of all health outcomes, and Table 3 reports a description of estimated marginal means and confidence interval values, both reported across all study sessions.
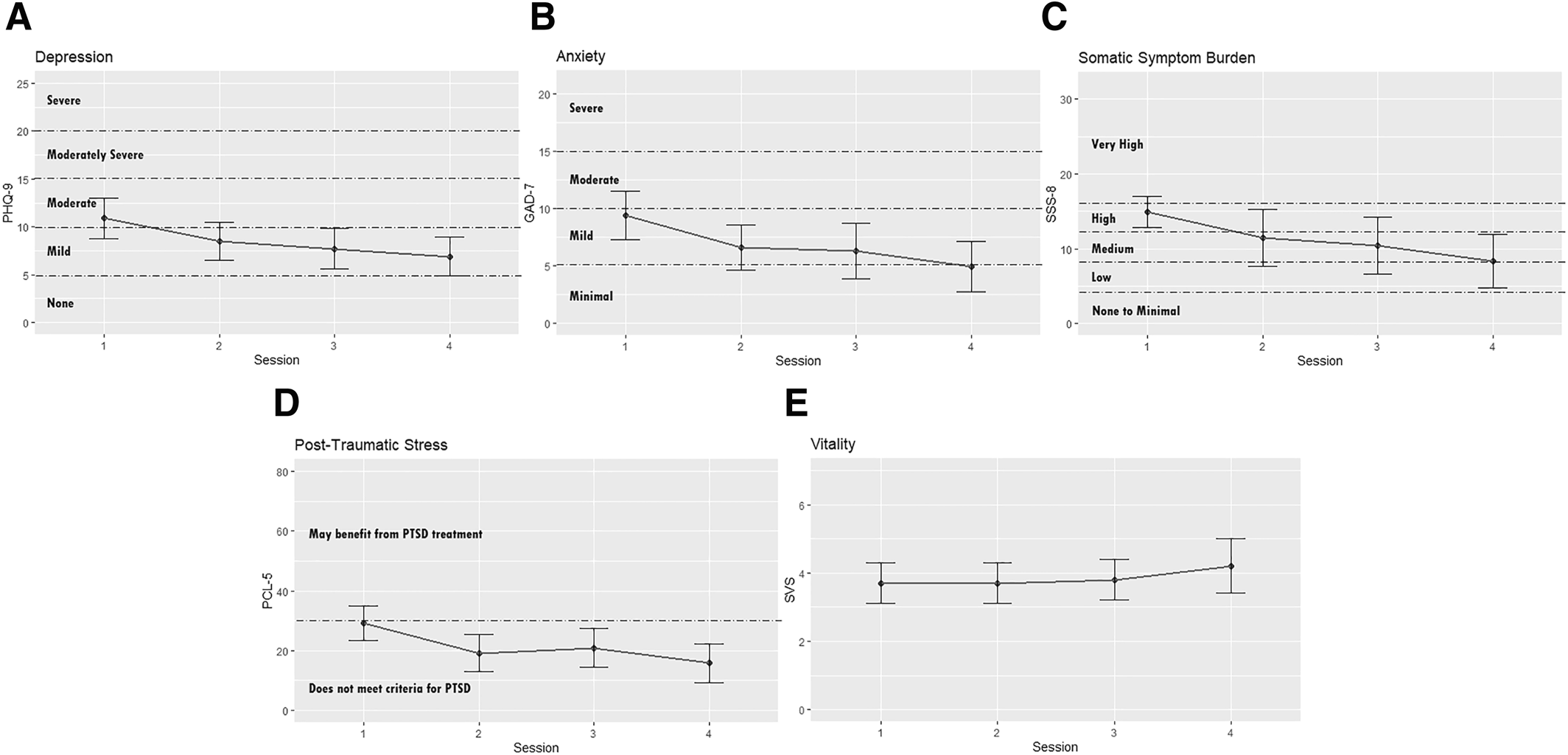
FIGURE 2. Estimated marginal means for health outcomes across HMR sessions. A. Depression. B. Anxiety. C. Somatic Symptom Burden. D. Post‐Traumatic Stress. E. Vitality. Note: For the PHQ‐9, GAD‐7, SSS‐8, and PCL‐5, each clinically significant level is indicated with a dashed line. Graphs were individually scaled to the theoretical range of the validated psychometric tool used to measure the health outcome. GAD‐7, Generalized Anxiety Disorder‐7; PCL‐5, PTSD Checklist for DSM‐5; PHQ‐9, Patient Health Questionnaire; SSS‐8, Somatic Symptom Scale.
| Variable | Mean (95% CIa) | p‐value | |||
|---|---|---|---|---|---|
| Session 1 | Session 2 | Session 3 | Session 4 | ||
| Depression | 10.9 (8.7, 13.0) | 8.5 (6.5, 10.5) | 7.7 (5.6, 9.7) | 6.9 (4.9, 8.8) | 0.05* |
| Anxiety | 9.4 (7.3, 11.5) | 6.6 (4.6, 8.6) | 6.3 (3.9, 8.8) | 4.9 (2.7, 7.1) | 0.03** |
| Symptom burden | 14.9 (12.7, 17.2) | 11.4 (8.6, 14.2) | 10.4 (7.6, 13.1) | 8.3 (5.7, 11.0) | <0.01** |
| Post‐traumatic stress | 29.2 (23.5, 34.8) | 19.2 (13.0, 25.3) | 20.9 (14.4, 27.4) | 15.8 (9.4, 22.3) | 0.01** |
| Vitality | 3.7 (3.1, 4.3) | 3.7 (3.1, 4.3) | 3.8 (3.2, 4.5) | 4.2 (3.4, 4.9) | 0.72 |
All health outcomes yielded a directionally positive result over time. Levels of depression, anxiety, symptom burden, and PTSD symptoms decreased; vitality improved. Four were statistically significant: depression (p = 0.05), anxiety (p = 0.03), symptom burden (p < 0.01) and PTSD symptoms (p = 0.01).
Individual somatic symptoms improved among 67%–95% of participants experiencing a given symptom at baseline: 79% improved stomach or bowel problems, 89% improved back‐related pain, 95% improved arm‐, leg‐, and/or joint‐related pain, 70% improved head‐related pain, 81% improved chest‐related pain or shortness of breath, 67% improved dizziness, 94% improved feeling tired and/or having low energy, and 92% improved trouble sleeping.
DISCUSSION
This study is the first known to report outcomes of the use of HMR® as a feasible intervention and suggests significant improvements in anxiety, somatic symptom burden, and PTSD associated with chronic pain and its associated symptoms. These are notable outcomes in a population that has often sought multiple providers and treatments to manage their symptoms.
Demographically, women were more likely to participate in our study than men, consistent with other MBT studies (30). Regarding ACEs, almost 100% had at least one ACE; 31 participants (over 50%) experienced 4 or more which places them at high risk to develop toxic stress response and associated chronic illness (15).
When comparing our study to other MBTs for chronic pain in the literature, we have chosen to review only systematic reviews and meta‐analyses of mind‐based therapies without somatic movement due to the plethora of studies that exist. A Cochrane review of 75 studies (n = 9401 participants) examined CBT, behavioral therapy (BT), and acceptance commitment therapy (ACT) for chronic pain, and found a small benefit for CBT, but no evidence was demonstrated for BT or ACT (11). An AHRQ review of 233 randomized controlled trials (RCTs) with similar population parameters to our current study (low back pain [LBP] and fibromyalgia [FM]) revealed CBT was associated with small improvements in LBP short‐term (−0.75 per a 0–10 scale, 1 to <6 months post‐MBT), intermediate‐term (−0.71, 6–12 months post‐MBT), and long‐term (−0.55, >12 months post‐MBT) whereas mindfulness‐based stress reduction (MBSR) demonstrated small improvements intermediate‐term. For FM, CBT was associated with a small improvement in pain compared to a control arm short‐term (−0.62) but not intermediate‐term. No clear short‐term reductions of function and pain were found for MBSR short‐term, but small improvements were noted immediate‐term. Our study suggests consistency with these findings. Pain, which was part of somatic symptoms, was aggregately reduced from a very high to medium range by the end of the study period.
Regarding emotional symptoms, 11 systematic reviews with 20 meta‐analyses reported a small to moderate benefit for mind‐based interventions (meditation and mindfulness) on depression in patients with chronic pain (9). The sample was similar to ours in that the majority of participants were middle‐aged women. Interventions had a small to moderate effect on depression in chronic pain conditions. Depression and symptoms of anxiety, a common component of depression, both significantly decreased in our study.
PTSD, which often co‐occurs with chronic pain, was found in a sizable proportion of participants in our study. MBTs are widely recommended in the treatment of PTSD and chronic pain (31). One study using Eye Movement Desensitization and Reprocessing (EMDR), a commonly used MBT for PTSD, noted 50% of patients had improved anxiety and PTSD symptoms compared to 0% of patients in the control group (32). The significant improvement in PTSD symptoms reported in our study warrants a RCT to confirm results.
While we have tried to compare our study to other MBTs without somatic movement, it is important to note that HMR® is not the same type of intervention, rather it embraces holonomic philosophy. “Holonomic” is derived from Karl Pribram's work in which memory in the physical body is not distributed equally as would occur in a holographic system but is evidenced as more site‐specific in the human body (33). This enables the HMR® practitioner to target a specific memory at a specific site, which is unlike any other therapy.
The practitioner employs a clean‐language approach, empowering the participant to use their personal internal language to gently access and transmit proof of safety to the specific moment and site of trauma encoding. Since every moment of trauma encoding involves a unique set of personal frequencies captured when consciousness is bound or paused, the participant alone can determine the missing frequencies or colors that restore safety. Early childhood developmental safety may be established via sight, sound, or olfaction even before language is established. Focus on the holonomic fragment of a memory provides access to the whole memory. The participant is offered resources to provide expedient assistance in resolving psychological and physiological distress in the effort to reduce state‐bound symptoms without re‐live or abreaction.
EMDR has proven effective in reducing the impact of adult memory but is less effective with childhood memory. Since HMR® creates sufficient safety to enable the participant to maintain dual states of consciousness, the adult's vocabulary can be used to access, articulate, and address the pain of the wounded parts. HMR® is more participant‐centered and body‐centered than thought field therapy/emotional freedom technique or energy psychology approaches in that it does not depend on the therapist's guidance or utilization of pre‐determined neural pathways or meridians but allows the participant's own internal mapping process to dictate the proper site of access and resolution. Since the conscious, rational, moral mind is recognized as 5% of consciousness, HMR® targets the 95% subconscious mind where trauma imprints and is stored.
While CBT has proven helpful in addressing the cognitive and behavioral impact of trauma, HMR® focuses on effective methods to discharge the affect or emotion which binds one to trauma or abuse and, therefore, was termed an “emotional reframing” technique, versus a cognitive approach. Individuals who perseverate with past traumatic events are often initially emotionally overwhelmed, which then produces subsequent cognitive and behavioral effects. While CBT helps a participant to become aware of inaccurate or negative thinking, HMR® seeks to address the affective trauma which precipitates, underlies, and results in adverse thought forms and behavioral coping mechanisms. That which attaches us to memory and the adverse effect of trauma is the emotional charge, which binds us to adverse thoughts. HMR® was designed to address and to help resolve the emotional attachments created through trauma and overwhelm. By remaining participant‐centered, body‐centered, and utilizing a strict clean‐language approach with no authoritative hypnotic induction employed, the possibility of false memory introjection for the participant is avoided.
Limitations
Several limitations are acknowledged in this study. First, this was a feasibility study without randomization, or a comparison group. Second, the timing of sessions for the intervention was variable to accommodate patient schedules. Regardless of the session schedule, an overall improvement in symptoms was observed. Third, specific pain scores were not consistently captured. HMR® practitioners indicated participants experienced pain that was difficult to quantify, and as therapy ensued and memories surfaced, pain would emerge in other parts of the body not previously reported. Finally, this sample lacked racial and ethnic diversity; therefore, these findings may not generalize to other populations.
CONCLUSION
HMR® is feasible and suggestive of being a potential intervention for chronic pain and its accompanying symptoms. Now that feasibility and a signal for improvement were found, the next study should include the use of HMR® compared to a control group, possibly using a stepped‐wedge design to ensure that all individuals receive the intervention. Overall, HMR may be an effective bridge to the moment of memory encoding of trauma. Unlike other MBTs, HMR® participants use their own internal language for identification and resolution of the pain. The trauma imprinting can then be gently addressed, and the memory‐based components of pain resolved or reduced, which empowers participants to return to present‐time living with greater well‐being.
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33